Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 12 August 2021

Drug addiction: from bench to bedside
- Julian Cheron 1 &
- Alban de Kerchove d’Exaerde ORCID: orcid.org/0000-0002-0682-5877 1
Translational Psychiatry volume 11 , Article number: 424 ( 2021 ) Cite this article
21k Accesses
33 Citations
5 Altmetric
Metrics details
- Molecular neuroscience
Drug addiction is responsible for millions of deaths per year around the world. Still, its management as a chronic disease is shadowed by misconceptions from the general public. Indeed, drug consumers are often labelled as “weak”, “immoral” or “depraved”. Consequently, drug addiction is often perceived as an individual problem and not societal. In technical terms, drug addiction is defined as a chronic, relapsing disease resulting from sustained effects of drugs on the brain. Through a better characterisation of the cerebral circuits involved, and the long-term modifications of the brain induced by addictive drugs administrations, first, we might be able to change the way the general public see the patient who is suffering from drug addiction, and second, we might be able to find new treatments to normalise the altered brain homeostasis. In this review, we synthetise the contribution of fundamental research to the understanding drug addiction and its contribution to potential novel therapeutics. Mostly based on drug-induced modifications of synaptic plasticity and epigenetic mechanisms (and their behavioural correlates) and after demonstration of their reversibility, we tried to highlight promising therapeutics. We also underline the specific temporal dynamics and psychosocial aspects of this complex psychiatric disease adding parameters to be considered in clinical trials and paving the way to test new therapeutic venues.
Similar content being viewed by others

Remission from addiction: erasing the wrong circuits or making new ones?
Addiction as a brain disease revised: why it still matters, and the need for consilience

Neuroimaging biomarkers of addiction
Introduction.
Drug addiction including smoking, alcohol and illicit drug use is indirectly or directly responsible for 11.8 million deaths each year in the world [ 1 ]. According to the Global Burden of Disease study, this number is higher than deaths from cancer and accounts for a fifth of all deaths around the world [ 1 ].
Drug addiction is defined as a chronic, relapsing disease that results from the prolonged effects of drugs on the brain. Similarly to other neuropsychiatric diseases, drug addiction is intermingled with behavioural and social aspects that are equally important parts of the disease, complicating the overall therapeutic approach. Actually, it is only recently, in the beginning of the 21st century, that we started to see “the drug-addict” as someone suffering from a disease and whose brain has been altered fundamentally by drugs [ 2 ]. Therefore, the most effective treatment approaches include biological, behavioural and social-context components. Based on the latest scientific advances, treatment and management of drug addiction patients point towards a personalised strategy [ 3 ]. However, there are very few objective and effective strategies for treating drug addiction. Without the mandatory mechanistic basic knowledge on drug addiction, the development of new therapeutic strategies is postponed.
The neurobiological circuits and mechanisms that support compulsive seeking and consumption of drugs with addictive potential are partially known. They comprise a progressive shift in the involvement of ventral to dorsal and medial to lateral striatal circuitry [ 4 , 5 ], along with molecular and cellular adaptations to drugs of abuse exposure. They include neuronal and synaptic plasticity and modifications in gene expression, in part through epigenetic mechanisms [ 6 ]. Notably, drug-induced neuronal modifications can also occur in non-pathological processes, underlying the fact that drugs of abuse hijack normal adaptive changes in the brain [ 7 ]. Indeed, laboratory and clinical observations suggest that addiction is driven by the usurpation of neuronal processes that normally serve reward-related learning and memory. Most of the modifications that have been shown to be involved in a state of addiction (modified gene transcription, epigenetics, neuronal plasticity and neurotrophic mechanisms) are also associated with physiological forms of behavioural memory in murine model such as spatial memory, fear conditioning and operant conditioning [ 7 , 8 ].
We know that only a proportion of individuals (depending of the drug type) will develop drug addiction after several exposures [ 9 ]. This individual vulnerability is probably linked to both genetic and environmental factors [ 10 ]. Drug addiction is highly polygenic, as hundreds of genetic variations combined result in variable vulnerability [ 11 , 12 ]. Several types of environmental factors have been characterised and interact with an individual genetic background [ 12 , 13 ]. Psychosocial stress is one of the factors, but the most important one, is by far, the exposure to drugs of abuse. Usually, drug abuse starts with a ‘gateway’ drug (mostly socially driven) catapulting the individual vulnerability to other drugs of abuse [ 14 ].
During the last three decades, combine effort has been dedicated to identify brain regions and molecular pathways involved in the development of addiction to drugs of abuse. Here, we will focus on experimental approaches that helped to provide a clearer picture on the physiopathology of drug addiction guiding therapeutic opportunities.
Converging actions on brain reward pathway elicit its remodelling
The circuit at the centre of the disease is the mesolimbic pathway, also referred as the reward pathway (Fig. 1 ). The mesolimbic pathway includes dopaminergic neurons in the ventral tegmental area (VTA) of the midbrain and their targets in the limbic forebrain, especially the nucleus accumbens (NAc), a major component of the ventral striatum. The GABA medium-sized spiny neurons (MSNs, ~95% of striatal neurons), which are targets of glutamatergic and dopaminergic inputs, form two pathways [ 15 ]. The dopamine D1 receptor–positive (D1R) striatonigral MSNs project to the medial globus pallidus and substantia nigra pars reticulata (direct pathway) and coexpress dopamine D1 receptors and substance P, whereas D2R striatopallidal MSNs project to the lateral globus pallidus (indirect pathway) and coexpress dopamine D2 receptor, adenosine A2A receptor and enkephalin [ 16 , 17 ]. Through different initial mechanisms, drugs of abuse increase the release of dopamine in the NAc from the VTA [ 18 , 19 , 20 ]. This VTA-NAc pathway could be seen as primum movens for the acute rewarding effects of all drugs of abuse. Regardless that drugs of abuse have distinct protein targets and mechanisms of action, in the end, the main addiction-related modifications are common to nearly all drugs of abuse and converge on the VTA and NAc with common acute functional effects [ 21 ]. It is schematically conjectured, that when stimulated by dopamine, cells in the NAc produce feelings of reward and satisfaction [ 22 ]. The physiological function of this response is to facilitate the motivation for basic biological goal-directed behaviours as survival, social interaction and reproduction. By artificially causing a build-up of dopamine in the NAc, drugs of abuse generate an artificial reward effect [ 22 ]. As all drugs of abuse increase dopaminergic transmission to the NAc after acute administration, they also produce shared modifications in the mesolimbic system after chronic exposure. They include (i) hypofunction of the dopamine pathway that is seen as a major contributor to the negative emotional symptoms associated to drug withdrawal, leading to drug craving and relapse, and (ii) drug-induced adaptations in glutamatergic afferents to the NAc [ 23 , 24 ]. Clearly, these modifications in the mesolimbic system after the exposure to drugs of abuse is oversimplified. The hypofunction of dopaminergic system hypothesis is self-fulfilling in that research work has principally focused on dopamine to the exclusion of other neurotransmitters. Actually, some drug of abuse reinforcement appears to be independent of the mesocorticolimbic dopamine system (e.g. opioids [ 25 ], nicotine [ 26 ]), but support self-administration by imitating the effect of dopamine in the nucleus accumbens [ 21 , 27 , 28 ].
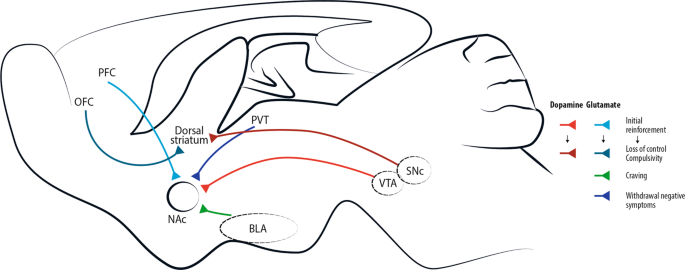
Addictive drugs of different types have a common effect of increasing levels of dopamine released by neurons projecting from the ventral tegmental area (VTA). This effect is central for initial drug reinforcement. Notably, drug taking with initial reinforcement involves a potentiation of the projection from prefrontal cortex (PFC) to nucleus accumbens (NAc), while other glutamatergic projections are mostly involved in craving, like basolateral amygdala (BLA)-NAc projection, or in withdrawal/negative symptoms, like paraventricular thalamus (PVT)-NAc projection. With increasing administration of drugs of abuse and progressive shift toward compulsive abuse, the dorsal (dorso-lateral) striatum seems more and more implicated, with dopaminergic cells involved shifting progressively from the VTA to the substantia nigra pars compacta (SNc) [ 4 ]. Recently, data acquired through optogenetic dopamine neuron self-stimulation suggested prominent synaptic strengthening of the orbitofrontal cortex (OFC) to dorsal (dorso-medial) striatum projection in compulsive mice [ 31 ].
Drug addiction is conceptually defined as a three-stages cycle: (1) consumption/binge/intoxication, (2) withdrawal with its negative affect and (3) craving stage (Fig. 2 ) [ 27 ]. Animal models and human imaging studies have exposed the different brain areas involved in each of these stages. Briefly, the VTA-NAc (for reinforcement) and dorsal striatum (for stimulus-response habits) are important for the consumption/binge/intoxication stage, the extended amygdala with the hypothalamus and the brainstem in the withdrawal stage and cortical areas, the dorsal striatum, the hippocampus and the basolateral amygdala in the craving stage (Fig. 2 ).
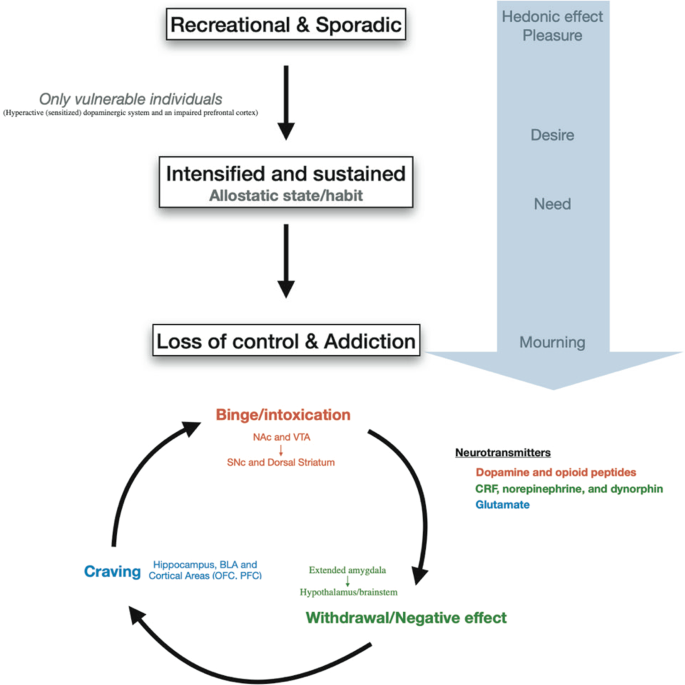
Progression to addiction is defined as a transition between three consecutive phases [ 252 ]: (1) Recreational, sporadic drug taking, in which drug of abuse administration is occasional and one activity among many other distractions of the individual. (2) Intensified and sustained drug use, in which drug administration strengthens and becomes the principal recreational activity of the individual; at this phase drug taking becomes a habit. (3) Loss of control of drug use and addiction, in which drug seeking and taking are now the principal activity of the patient. The first phase can occur to every person as drugs of abuse hijack the same brain circuit as natural rewards. The second phase occurs only in vulnerable users. The phase of addiction seems to be due to a second vulnerable trait with loss of control and compulsivity. Three stages of addiction are described [ 27 ]: (1) Binge/intoxication stage: reinforcing effects of drugs may initially use mainly dopamine and opioid peptides in the nucleus accumbens (NAc) and involves the ventral tegmental area (VTA). Subsequently, cue–response habits develop and includes the substantia nigra pars compacta (SNc) and the dorsal striatum. (2) Withdrawal/negative affect stage: the negative emotional state of withdrawal may involve the extended amygdala with corticotropin-releasing factor (CRF), norepinephrine and dynorphin as key neurotransmitters. Main projections of the extended amygdala consist of the hypothalamus and brainstem. (3) Craving stage: this stage includes conditioned reinforcement in the basolateral amygdala (BLA) and contextual processes in the hippocampus. This is controlled by cortical areas (prefrontal cortex (PFC) and orbitofrontal cortex (OFC)). A key neurotransmitter involved in the craving stage is glutamate.
The progression of drug addiction begins with the first exposure, mostly when the drug is taken voluntarily for its recreational and hedonic effect, and progressively consolidates during repeated but still controlled drug use. While administration intensifies along with loss of control over drug intake, drug use becomes habitual and compulsive in vulnerable individuals [ 4 , 29 , 30 ] (Fig. 2 ). This progression from voluntary drug intake to habitual and compulsive use represents a progression from ventromedial to more dorsolateral regions of the striatum and from prefrontal cortex (PFC) to orbitofrontal (OFC) and more global cortical region [ 4 , 31 ] (Fig. 1 ).
Synaptic plasticity
Brain plasticity is a fascinating capacity allowing appropriate modification of the neural activity in response to new experiences and environmental stimuli [ 32 ]. Modifying the synaptic strength between neurons is widely assumed to be the mechanism by which memory is encoded and stored in the brain [ 7 ]. Hence, it is appealing to hypothesise that drugs of abuse cause long-term alterations on behaviour by changing synaptic plasticity in key brain circuits [ 4 , 7 , 32 ].
Drugs of abuse such as cocaine induce specific synaptic plasticity in the mesolimbic circuitry. One single injection of an addictive drug can already modify the excitatory synaptic strengths in the VTA. Indeed, it has been extensively shown that the AMPA/NMDA ratio is increased in VTA dopamine neurons after one dose of cocaine and that some glutamate AMPA receptor 2 (GluA2)-containing AMPA receptors (AMPARs) are exchanged for GluA2-lacking ones [ 33 , 34 ]. At the same time, NMDA receptor (NMDAR) function decreases. All these elements cause an impairment in eliciting long-term potentiation (LTP). Different types of synaptic plasticity in VTA dopamine neurons induced by rewarding and aversive experiences are comprehensively reviewed by Pignatelli and Bonci [ 35 ]. Midbrain dopamine neurons are central in the mesolimbic circuitry for both natural rewards and drugs of abuse [ 18 , 36 ]. The VTA is known to be a central hub integrating numerous inhibitory inputs as GABAergic synapses represents 50–80% of all synapses onto VTA dopamine neurons [ 37 , 38 , 39 ]. GABAergic inhibition of dopamine neurons is mediated by both fast ionotropic GABA A receptors and slow metabotropic GABA B receptors [ 40 ].
In 2017, Edwards et al. [ 41 ] showed that the principal monosynaptic projection to VTA dopamine neurons arising from the NAc [ 42 ] inhibits the firing of dopamine neurons via activation of GABA B receptors, whereas local VTA inhibitory interneurons inhibits dopamine neurons through GABA A receptors. Today, it is well established that pharmacological activation of GABA B receptors (e.g. by baclofen) reduces cue-associated cocaine craving as well as reduce cocaine use in humans [ 43 , 44 , 45 ] and it reduces rewarding and reinforcing effects of cocaine on animal models [ 46 , 47 , 48 , 49 ]. Edwards et al. report [ 41 ] indicates that the therapeutic effects of baclofen might pass through VTA dopamine neurons’ GABA B receptors. Intrathecal Baclofen is an effective and safe long-term treatment used worldwide to treat severe spasticity [ 50 , 51 ]. Oral baclofen is less effective and has significant rates of side effects, like sedation, somnolence, vertigo and headache especially when prescribed off-label for drug addiction (because higher doses are commonly used) [ 51 , 52 ] . . Indeed, contrasting results on the effect of baclofen in reducing alcohol craving [ 53 , 54 , 55 ] and cocaine dependence [ 43 , 56 ] were probably due to different severity of alcohol dependence of the enroled patients. This is way higher dose are tested and often prescribed off-label for drug addiction [ 55 ]. Thus, self-poisoning that could lead to severe toxicity and death represents one the major concern of baclofen use in drug addiction. Therefore, baclofen should be prescribed with caution and close monitoring [ 52 , 57 ].
Together with drug of abuse-induced LTP at excitatory synapses, plasticity of GABAergic inhibitory synapse in the VTA also have an impact on the firing rate of VTA neurons, at least following opioid [ 58 ] and cocaine administration [ 59 ]. Normally, NMDA activation, during excitatory LTP (induced by high-frequency stimulation), leads to the release of NO that will activate guanylate cyclase in adjacent GABAergic terminals, which in turn, leads to increase in GABA release. This presynaptic NMDA receptor-dependent GABAergic LTP heterosynaptic plasticity, is named LTP GABA . Nugent el al. [ 58 ] showed that opioids blocks LTP GABA through a disruption of the coupling between nitric oxide (NO) and guanylate cyclase. The incapability of GABAergic synapses to potentiate after morphine or cocaine administration may promote LTP of glutamatergic synapses [ 58 , 59 ]. The early loss of inhibitory control combined with potentiation of glutamatergic synapses on dopaminergic neurons might represent adaptations that increase vulnerability to addiction [ 58 , 59 ]. Furthermore, GABA A receptor modulators modify the addictive drugs effects [ 60 , 61 ], and targeting these receptors might be seen as an effective therapeutic strategy but precluded by many side effects among which dependence itself [ 62 , 63 , 64 ].
In addition to the discovery of LTP GABA , Nugent’s group showed that morphine is also able to modulate a form of postsynaptic LTD (LTD GABA ) at GABAergic synapses onto VTA dopamine neurons. Remarkably, after a single administration of morphine, LTD GABA was absent in slices from morphine-treated rats while unaffected in slices from saline-treated rats, indicating a bidirectional control of morphine on GABAergic synaptic plasticity in the VTA [ 65 ]. This absence of LTD GABA is suggested to result from an occlusion effect due to prior morphine-induced decrease in GABAergic synaptic strength through potentiation of glutamatergic transmission and mediated by endocannabinoid signalling [ 66 ]. It is also possible that morphine alters the ability of synapses to exhibit evoked LTP or LTD in the VTA. Previous experiences such as exposure to drugs of abuse, stress, visual or sensory deprivation can change the ability of synapses to undergo subsequent plasticity in response to LTP and LTD induction protocols. This concept of modification of plasticity capability is referred as metaplasticity [ 67 ].
In the NAc, chronic exposure to addictive drugs induces specific synaptic changes that are different from those of the VTA, including a decrease of the AMPA/NMDA ratio as some AMPARs are endocytosed. This leads to a depressed synapse (sometimes referred as long-term depression (LTD) like state), where NMDAR-dependent LTD is reduced or, in some experiments, abolished [ 68 , 69 ]. Highlighting the importance of temporal aspects, studies of withdrawal period after chronic administration of cocaine, showed that synaptic AMPAR levels increase during the first week of withdrawal and persist elevated for weeks [ 70 , 71 , 72 ]. It is established that cocaine challenge transiently decreases AMPAR surface expression, while AMPARs recover back to upregulated levels within a week, with a continuous increase during what is known to be the incubation of craving stage [ 73 ].
The abstinence period after withdrawal is of particular interest considering the classical progression of the disease, the chance of relapse and the opportunity for new therapeutic targets. A seemingly counterintuitive concept named ‘incubation of cocaine craving’ was introduced by Grimm et al. [ 74 ] who modelled cocaine-craving behaviour by using rats trained to press a lever to receive an injection of cocaine and were then forced in a withdrawal period where cocaine reward was no longer given. This concept of ‘incubation’ did not originate in drug addiction research but came from a four-stage model of the creative process proposed by Graham Wallas in 1926 [ 75 ]. Consistent with clinical observations in humans [ 76 , 77 , 78 ], they showed that relapse was progressively stronger over 2 months of cocaine withdrawal and suggest that a craving syndrome progresses or ‘incubates’ during the first 2 months of cocaine abstinence, and probably lasts for longer [ 74 ]. Subsequently, it was shown that this increase was due to the addition of new AMPARs lacking GluA2 and that these new receptors mediate the ‘incubation of cocaine craving’ [ 72 ]. Conrad et al. [ 72 ] showed that after extended withdrawal from cocaine, addition of synaptic AMPARs together with the increased conductance of GluA2-lacking AMPARs triggers higher sensitivity of NAc neurons to cocaine-related cues, leading to a strengthening of drug craving syndrome and relapse. In line with these results, it was suggested that as soon as abstinence is reached, the risk of relapse might be reduced if GluA2-lacking AMPARs were inactivated or removed from NAc synapses. It was thus proposed that GluA2-lacking AMPARs could be a new target for drug development for the treatment of cocaine addiction. While these calcium permeable AMPARs are also critical for the pathogenesis of numerous other neurological disorders (including epilepsy [ 79 ], fragile X syndrome [ 80 ], amyotrophic lateral sclerosis [ 81 ], Parkinson’s [ 82 ] and Alzheimer’s [ 83 ] diseases), developing drugs that specifically target them and not calcium-impermeable AMPARs, which are critical for normal CNS function, is challenging [ 84 ] (Fig. 3 ).
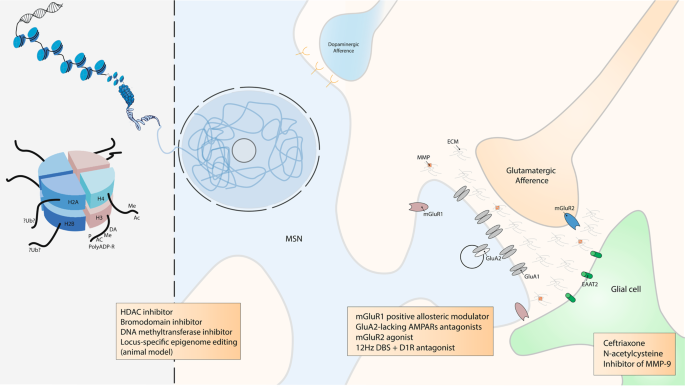
DNA is packaged inside nuclei with the help of histones. These are positively charged proteins that strongly adhere to negatively charged DNA and form complexes called nucleosomes. Each nucleosome is composed of DNA wound around histone octomers (H2A, H2B, H3 and H4). Nucleosomes fold up to form chromatin fibre, which forms loops compressed and folded to produce fibres, which are coiled into the chromatid of a chromosome. Only by loosening compacted chromatin, the DNA of a specific gene can be made accessible to transcription. Some of these drug-induced modifications at the chromatin level are extremely stable and sustain the drug of abuse-induced long-term behaviours. Among them, histone post-translational modifications (PTMs) are known to be causally involved in drug-induced behaviours [ 194 ]. PTMs include acetylation (Ac), methylation (Me), phosphorylation (P), ADP ribosylation (PolyADP-R) and dopaminylation (DA), among a growing list of newly discovered modifications [ 162 , 172 ]. For example, while ubiquitylation (Ub) of H2A is known to be a key interactor of H3 methylation [ 253 ], its supposed role in drug addiction is still unknown. At this epigenetic level, some drugs were demonstrated to have an influence on drug-induces behaviours such as histone deacetylase (HDAC), bromodomain and DNA methyltransferase inhibitors. Locus-specific epigenome editing is now encouraging as a new field of investigation as it might help to the discovery of new specific and causal drug of abuse targets. Overview of the tetrapartite glutamatergic synapse composed of a medium spiny neuron (MSN), a glutamatergic projection, a glial cell and the extracellular matrix (ECM). Here, we focused on synaptic potentiation after drug of abuse administration with the addition at the post-synaptic membrane of glutamate AMPA receptor 2 (GluA2) lacking AMPA receptors (AMPARs). This mechanism might be reduced by metabotropic glutamate receptor 1 (mGluR1) positive allosteric modulator or more directly by GluA2-lacking AMPARs antagonists. In the same way, it was also shown that presynaptic mGluR2 agonists can potentially abolish drug seeking and impair craving incubation. Optogenetically-inspired 12 Hz deep brain stimulation (DBS) in the nucleus accumbens can also be a promising novel therapeutic for addiction. Finally, ceftriaxone, N-acetylcysteine, and inhibitor of matrix metalloproteases 9 (MMP-9), mainly through their action on glial cell and the ECM, are very interesting molecules that may be added in the addiction therapeutic arsenal.
Inspired by previous work performed in the VTA showing that metabotropic glutamate receptor 1 (mGluR1) LTD induces removal of GluA2-lacking AMPARs from synapses [ 33 , 34 ], Loweth et al. [ 85 ] demonstrated that synaptic GluA2-lacking AMPAR decrease could be accomplished by in vivo evoked mGluR1 LTD in the NAc. More importantly, their group showed that after prolonged cocaine or methamphetamine withdrawal, systemic injection of a mGluR1 positive allosteric modulator attenuated the expression of incubated craving by reducing GluA2-lacking AMPARs in the NAc [ 85 , 86 ]. These results suggest a strategy in which abstinent methamphetamine or cocaine users could use a systemically active compound to protect themselves against cue-induced relapse.
These latter studies were conducted without differentiating between D1 receptor D1R MSNs and D2R MSNs. In 2014, Pascoli et al. [ 87 ] demonstrated that this increase in the strength of excitatory afferents was exclusively related to D1R MSNs. Interestingly, the type of drug-evoked plasticity involved is also dependent on the input. It has been shown that even in the same D1R MSN a synapse connecting the PFC to the NAc increases its strength by inserting GluA2-lacking AMPARs whereas a synapse connecting the ventral hippocampus to the NAc increases the AMPA/NMDA ratio by inserting GluA2-containing AMPARs [ 87 ].
Besides operant self-administration, all these long-term synaptic modifications also underlie behavioural changes associated with drugs of abuse, such as locomotor sensitisation [ 88 , 89 ]. Locomotor sensitisation is a behavioural protocol used to model drug-induced behaviour [ 90 , 91 ]. In rodents, repeated cocaine injection induces gradually increased locomotor activity; after 5 days of consecutive injections, the locomotor response reaches a ceiling level. This state lasts for months after cocaine withdrawal [ 91 ]. As an experimental model, locomotor sensitisation is linked with increased tendency to self-administer psychostimulants [ 92 , 93 ] and with reinstatement of previously extinguished self-administration [ 94 , 95 ]. Whereas the existence of psychomotor sensitisation in humans is discussed [ 96 , 97 ], it is a key aspect of vulnerability to drug addiction and relapse, specifically drug craving or compulsive drug-seeking behaviour [ 91 , 98 , 99 ]. Still, locomotor sensitisation can be dissociated from the rewarding effect of a drug of abuse and conditioned place preference or self-administration are more appropriate experimental paradigms to test this aspect [ 100 , 101 , 102 ]. Even if drug-induced locomotor sensitisation is unclearly present in humans, as an animal model it offers a clear readout to understand the mechanisms by which drugs of abuse induce long-term brain modifications [ 91 ].
Furthermore, it has been elegantly demonstrated that optogenetic stimulation of the excitatory projections to the NAc is able to reverse cocaine and alcohol-evoked plasticity [ 87 , 88 , 89 ]. Briefly, applying a NMDAR or mGluR1-dependent LTD on cortico-accumbal glutamatergic synapses, before a drug of abuse administration, diminishes its effect. In another study, Luscher’s team took advantage of the knowledge, obtained from optogenetic in vivo experiment in rodents, to implement a novel deep brain stimulation (DBS) protocol that abolishes behavioural sensitization to cocaine (and thus that would be efficient during the relapse phase) [ 103 ]. Basically, the idea is to manipulate synaptic plasticity in the NAc to reverse pathological synaptic transmission and its associated behaviours following exposure to drugs of abuse. In this study, as a therapeutic use of optogenetic tools in humans is for now inapplicable [ 104 ], the authors reversed cocaine-evoked plasticity and thus drug-induced behaviours by using DBS instead of optogenetic. Indeed, DBS is routinely used in clinic and a new DBS protocol can easily be translationally implemented to the human therapeutics [ 105 , 106 ]. They refined the classical high-frequency DBS protocol (that has no sustained effect on cocaine sensitization, probably because it does not affect synaptic plasticity) by applying a low frequency stimulation (12 Hz to equal the one used in the optogenetic endocannabinoid- dependent LTD protocol) in the NAc together with the administration of a D1R antagonist necessary to unmask the mGluR-dependent LTD in D1R MSNs as demonstrated previously [ 107 ] (Fig. 3 , see section on clinical treatment for broader discussion on DBS).
Kalivas’ group showed in 2009 [ 108 ] that after extended withdrawal from chronic cocaine self-administration, cocaine-induced metaplasticity at the excitatory synapses in the NAc that impairs the ability of PFC stimulation to produce LTP or LTD in NAc MSNs. They also showed that N-acetylcysteine reverses cocaine-induced metaplasticity, allowing the induction of both LTP and LTD and that N-acetylcysteine decreases cocaine-relapse in a rodent model. We are currently awaiting the results of a randomised and control study that is testing newly detoxified (and therefore abstinent) hospitalised patients who received a 3–4 week course of treatment, in order determine if N-acetylcysteine can be a useful medication candidate to avoid relapse in patients with cocaine dependence (NCT03423667).
GABAergic D1R and D2R MSNs, equally compose and are mosaically intermingled throughout the striatum [ 109 ]. As explained above, D1R and D2R MSNs send axonal projections outside the striatum, forming the two main output pathways, respectively the direct and indirect pathways [ 16 , 17 ]. In a certainly oversimplified model, the activation of the D1R MSNs result in facilitation of locomotion, reward, and reinforcement while the activation of D2R MSNs result in opposing effects [ 110 , 111 , 112 , 113 ]. In addition to the long-range projections, these neurons form short-range synaptic connections with one another within the striatum, and because they consist of inhibitory collaterals, a mechanism known as lateral inhibition [ 114 , 115 , 116 , 117 ]. Interestingly, these connections are not symmetrical, with D2R MSNs forming more synaptic connections on D1R MSNs [ 115 , 117 ]. Through this previously understudied collateral transmission, Dobbs et al. [ 115 ] presented a novel mechanism by which cocaine exerts its stimulant effect: cocaine, by blocking DAT receptors enhance levels of dopamine and subsequently activating D2Rs, causes a suppression of lateral inhibition and thus disinhibition of D1R MSNs in the NAc which in turn promotes locomotion [ 115 ]. Furthermore, Alvarez’ group suggested that constitutive low D2R levels, through imbalanced lateral inhibition, might pre-sensitised D1R MSNs, facilitate behavioural plasticity to repeated cocaine and promotes an addiction vulnerable phenotype [ 116 ].
The characterisation of the role of glia and the extracellular matrix (ECM) in drug-induced synaptic plasticity is an exciting emerging field of drug addiction research as it comes with promising new therapeutic possibilitiess [ 118 , 119 , 120 ]. Mulholland et al. [ 118 ] summarised and emphasised the role of the ECM and of astroglial cells in the regulation of synaptic plasticity. Of great interest, restoring downregulated glutamate transporter 1 (EAAT2) with ceftriaxone reduces drug seeking in animal models [ 121 , 122 ]. Matrix metalloproteases (MMP) are important regulators of the ECM and contribute to synaptic plasticity [ 123 ]. Inhibiting their activity result in suppression of the reinstatement of cocaine conditioned place preference [ 124 ] and selectively inhibiting MMP-9 prevents cue- and cocaine-induced reinstatement of cocaine self-administration [ 119 ]; these results open additional therapeutic possibilities with the use of inhibitors of MMP-9 as an innovative targeted approach [ 119 , 124 , 125 ] (Fig. 3 ). Still, at our knowledge, there are no randomised controlled study currently investigating these ECM-related drugs.
Drugs of abuse-induced modifications in glutamatergic nuclei targeting the NAc, or the VTA and essential part of the reward circuit, are less studied than cortico-striatal synapses despite the fact that they play a crucial role in the development of drug addiction. Indeed, in the OFC and PFC, chronic alcohol exposure significantly increases LTP in pyramidal neurons [ 126 , 127 ]. Kazanetz et al. [ 128 ] showed that repeated cocaine injections impair endocannabinoid-LTD and mGluR2/3-LTD in the PFC. They postulated that this might mechanistically participate in the induction of a postsynaptic, observed LTP-like phenomenon with an enhanced AMPA/NMDA ratio. It was also demonstrated that neurons of the infralimbic cortex present a decrease in mGluR2 [ 129 ]. In addition, alcohol-dependent rats exhibit an escalation of ethanol seeking, which was abolished by restoring mGluR2 expression in the infralimbic cortex via viral-mediated gene transfer [ 129 ]. Notably, mGluR2 agonist was shown to impair the incubation of cocaine craving [ 130 ] and to attenuate reinstatement of cocaine-seeking [ 131 , 132 ](Fig. 3 ). Recently, Caprioli et al. [ 133 ] extensively reviewed preclinical studies on allosteric modulators of mGluRs on animal models of drug addiction and their potential translational implications. The results reviewed [ 133 ] indicate an remarkable effect of allosteric modulators of presynaptic mGluR2 and possibly mGluR7, supporting the idea that these compounds should be tested as potential medications for addiction treatments.
Besides the PFC, other brain regions appear to be key areas in drug addiction as the paraventricular thalamus (PVT) - a central hub for cortical, sensory and limbic information [ 134 , 135 , 136 , 137 , 138 , 139 , 140 ]. In 2016, Zhu et al. [ 141 ] showed that chronic morphine administration potentiates excitatory synapses between the PVT and D2R MSNs via insertion of GluA2-lacking AMPARs. Remarkably, in vivo optogenetic depotentiation at these synapses abolishes morphine withdrawal symptoms. In a recent paper, projections from the PVT to the NAc were shown to be critical for augmentation of heroin seeking in food-restricted rats [ 142 ] (Fig. 1 ). Actually, Otis et al. [ 143 ] demonstrated that the PVT is an integrative hub for reward seeking behaviour and that PVT-NAc neurons integrate different inputs from the PFC and the lateral hypothalamus to precisely guide reward seeking behaviour. In a recent review, De Groote et al. [ 140 ] focused on the new advances in the understanding of the roles of the PVT-NAc connections in motivated behaviours, highlighting their implications in drug addiction.
Drug addiction-related genes and transcriptomic regulation
Modifications in gene expression contribute to the long-lasting effect sustaining drug addiction; thanks to gene-expression arrays, RNA-sequencing and candidate gene approaches, the specific genes and their regulatory transcriptomic mechanisms involved in drug addiction development and maintenance are now better understood.
Drug addiction-related genes
For example, the use of conditional gene knockout in mice emphasises the importance of monoamine membrane transporters (dopamine transporter, and serotonin transporter) [ 144 , 145 ] and of mGluRs [ 146 , 147 ]. As new animal models of drug addiction, these approaches are also useful to better characterise fine-tuning of important pathways involved in addiction. For example, a scaffold protein known as Maged1 has been shown to be involved in cocaine reward and reinforcement [ 148 ]. We demonstrated that Maged1 inactivation impairs drug-evoked dopamine release and glutamatergic synaptic plasticity in the NAc. Inactivation of Maged1 in mice was able to abolish behavioural sensitization to cocaine as well as cocaine conditioned place preference and operant self-administration behaviours [ 148 ]. This sole genetic alteration, causally linked to a strong alteration of drug-induced behaviours, impairs (at least) two core neuronal mechanisms leading to addictive behaviours: (1) cocaine-evoked release of dopamine in the NAc and (2) NAc plasticity, with a reduced AMPA/NMDA ratio and a resistance to LTD. Actually, it seems that, after Maged1 inactivation, the excitatory synapses in the NAc shift to a depressed state. Our hypothesis is that, in line with the previously discussed in vivo optogenetic induced LTD, this impairment could be a key factor for the significant decrease in sensitization to psychostimulants [ 87 , 103 , 148 ]. Actually, it seems that placing neurons in a state of ‘presensitization’ is able to prevent drug-induced sensitization itself [ 148 , 149 ]. Our group is now trying to understand what are the cellular and molecular pathways directly altered by Maged1 inactivation and responsible for this strong anti-addictive drug phenotype. Remarkably, the promoter of Maged1 was found in a list of 213 promoters that co-precipitate with acetylated histones and with the activated form of cAMP response element binding protein (CREB) after chronic drug taking [ 150 ]. In line with this result, preliminary and unpublished results from our laboratory point out a specific epigenetic mechanism, in parallel with an alteration of synaptic plasticity in excitatory projection to the NAc, that would link Maged1 to its major effect on drug-induced behaviours. This selected gene approach is of great interest in refining our knowledge of pathways hijacked by addictive drugs. Using cell sorting of D1R MSNs and D2R MSNs as described previously [ 151 ], our group also identified the G-protein-regulated inducer of neurite outgrowth 3 (GPRIN3) in both MSN populations but strikingly more expressed in D2R MSNs [ 149 ]. The GPRIN family (GPRIN1, GPRIN2 and GPRIN3) are Gαi/o-regulated proteins suggested to intermediate the communication between GPCRs and the sequential intracellular target [ 152 ]. Indeed, GPRIN1 and GPRIN2 have been described as alternative (to adenylyl cyclase) mediators of GPCRs signalling but GPRIN3 had a much less defined role [ 152 , 153 ]. To understand the role of GPRIN3 in the pathophysiology of the D2R-indirect pathway, we induced a D2R-MSNs-specific knockdown (KD) of GPRIN3 using small hairpin RNA and lentiviruses [ 151 , 154 ]. We first observed a significant increase in distal branching, the points of convergence between glutamate and dopamine synapses in MSNs [ 155 ] and also key targets of cocaine, which itself promotes increase in distal branching in the NAc of mice [ 156 , 157 , 158 , 159 ]. Thus, we tested the cocaine acute effect and locomotor sensitization and observed a decrease in cocaine-induced hyperlocomotion after inactivation of GPRIN3 using a CRISP/Cas9 approach. The significant increase in distal branching in GPRIN3 D2R-MSNs KD corroborates our hypothesis that the lack of GPRIN3 induces a ‘presensitization process’, able to change the targets of cocaine and therefore altering its effects [ 149 ]. Finally, we provide the first evidence that GPRIN3 partners with D2R in the striatum and modulates cocaine-induced behaviours [ 149 ].
Transcriptomic and epigenetic regulations
Epigenetics is a broad field and has multiple definitions that comprise several biochemical mechanisms (including DNA methylation and histone modifications) sustaining modifications in gene expression throughout the lifecycle of an organism without mutations of the DNA itself [ 160 , 161 , 162 ]. Epigenetics can be considered as the process through which environment (and normal development) interacts with an individual’s genome to determine all phenotypic traits, in health and disease. Stable modifications in gene expression are also said to be ‘epigenetic’, because they are heritable in the short term (through mitosis) [ 160 ] and in some cases trans-generationally, thus, providing a potential mechanism for environmental influences to be passed from parents to offspring [ 163 , 164 , 165 ]. Handel and Romagopalan [ 163 ] mentioned that “epigenetics allows the peaceful co-existence of Darwinian and Lamarckian evolution”. Such trans-generational epigenetic inheritance of drug addiction vulnerability remains debatable [ 161 ], but has been increasingly studied for the last 20 years [ 166 , 167 ]. Some epigenetic changes are very stable, an thus mediate both drug addiction susceptibility and drug-induced brain alterations that underlie the development of drug addiction [ 161 ].
As the NAc is seen as the central hub of drug addiction, with the notion that chronic drug use induces long-lasting structural, electrophysiological and transcriptional changes in the NAc, researchers are mostly targeting epigenetic modifications in NAc cells. Still, considering initial reports of cocaine-induced epigenetic modifications [ 168 , 169 ], it might be relevant to study further epigenetic changes in other regions such as glutamatergic inputs to the NAc, and further in the VTA, as they are implicated in the physiopathology of drug addiction [ 170 , 171 ] as mentioned above.
To date, the three main epigenetic mechanisms consist of (1) DNA methylation, (2) action of the non-coding RNAs and (3) histone post-translational modifications (PTMs). As an illustrative example, we will focus here only on histone PTMs. PTMs of histone residues on their N-terminal tails, that protrude from the nucleosome core, control chromatin condensation and the switch between euchromatin and heterochromatin and thus DNA-accessibility and gene expressions. PTMs include acetylation, methylation, phosphorylation, ADP ribosylation, ubiquitylation and sumoylation, among a growing list of newly discovered modifications [ 162 , 172 ].
Among these PTMs, the most studied is the acetylation of H3 and H4, that is increased in the NAc after chronic exposure to drugs of abuse [ 150 , 173 , 174 ]. This increase in global acetylation levels is the result of drug-induced alterations in the balance of histone acetyltransferase and histone deacetylase (HDAC) function and is associated with gene activation. CREB-binding protein, a histone acetyltransferase critical to memory processes [ 175 ], is required for cocaine-induced increases in histone acetylation in the NAc [ 176 ].
Fifteen years ago, Tsankova et al. [ 177 ] showed that imipramine, a monoamine reuptake inhibitor used for decades to treat depression, was effective through histone remodelling in depression and highlight the therapeutic potential for chromatin regulation with histone methylation and deacetylation inhibitors in depression. Nevertheless, like with synaptic plasticity (see above), discovering a drug that would interfere with epigenetic mechanisms and thus decrease drugs of abuse effect faces temporal aspects issues [ 173 , 176 , 178 , 179 , 180 , 181 , 182 ]. Indeed, timing has a strong impact considering conflicting results obtained after experimental manipulations of histone acetylation. An acute administration of HDAC inhibitors systemically or directly into the NAc, promotes behavioural responses to the drugs. However, prolonged administration decreases cocaine behavioural effects. In 2013, adding a new layer of complexity, Kennedy et al. provided comprehension to this time-dependent regulation [ 183 ]. Remarkably, they showed that prolonged intraNAc administration (but not acute administration) of a HDAC inhibitor attenuated cocaine behavioural effects by inducing a form of repressive histone methylation. This study showed, for the first time, cross-talk among different types of histone modifications [ 183 ]. Besides cross-talk between different epigenetic modifications, multiple modifications work in parallel and there is often a decoupling between an observed modification at a specific locus and its final transcription [ 161 ]. Decoding these chromatin marks will be a future challenging field. Like with HDAC inhibitors, there are promising findings based on the use of DNA methyltransferases inhibitor [ 184 , 185 ] (Fig. 3 ). Though, the main issue with these new potential treatments for drug addiction is their lack of specificity. One of the key challenge for the pharmaceutical industry will be to generate small molecules with more specific targets [ 6 ].
While histone acetylation and methylation are increasingly studied, an important field of future investigation will be to understand the other drug-induced histone PTMs. It already seems that chronic cocaine alters levels of histone phosphorylation [ 174 , 186 , 187 ], and poly-ADP ribosylation [ 188 ]. Recently, an unexpected role for the intracellular dopamine in VTA has been revealed, showing that DA interacts with chromatin to initiate a new form of epigenetic regulation called dopaminylation [ 189 ] (see Table 1 for a summary of cocaine-related epigenetic modifications).
Further studies showed that histone PTMs that occur in the NAc after chronic drug administration are locus specific [ 150 , 190 , 191 ]. Even though, drugs of abuse alter global levels of multiple histone PTMs, such as increased histone acetylation or decreased methylation in the NAc, genome-wide studies have confirmed that a greater number of genomic sites show increased acetylation [ 150 ] or decreased methylation [ 190 , 191 ]. Conversely, hundreds of genes show opposite or no changes in these same PTMs after drug exposure. What defines whether, and in which direction, a specific gene is modified in the context of a global histone PTM is an intriguing and unsolved question [ 161 ]. These genome-wide studies (ChIp on chip or ChIpSeq) are nowadays fundamental to understand where PTMs and other epigenetic modifications are deposited. This will be fundamental to guide new therapeutics.
Actually, with new tools such as zinc finger proteins (ZFPs) DNA-binding domains and, more recently, RNA-guided CRISPR/dCas9 (drastically easier to design) [ 192 , 193 ], it is now possible to control epigenetic modifications at a single gene in a specific type of cell in a specific brain region [ 162 ]. Heller et al. demonstrated that gene-targeted epigenetic editing (targeted to the Fosb [ 194 ] and Cdk5 [ 195 ] locus with ZFP technology) can alter drug-related behaviours [ 194 , 195 ]. This represents crucial evidence that gene-specific changes to the epigenome are not simply correlated, but rather causal, in regulating transcriptional responses to drugs of abuse administrations. These new results of “causal epigenomics” are very encouraging as they open the way to precise translational therapeutic approaches for drug addiction and other CNS diseases.
Linking epigenetics and synaptic plasticity
Today, most studies investigate synaptic plasticity and epigenetic as two distinct fields and it is not clear how these research topics are connected to each other. Understanding how epigenetics is connected to synaptic plasticity is an emerging research issue [ 6 ].
Of course, bridging epigenetic mechanisms with synaptic plasticity is not limited to drug addiction field. For example, in 2011, Monsey et al. [ 196 ] elegantly demonstrated that DNA methylation and histone H3 acetylation regulate auditory fear conditioning and its related synaptic plasticity in the amygdala. In 2014, Massart et al. [ 197 ] suggested that sleep deprivation induces epigenetic modification (alteration in DNA methylation and hydroxymethylation) that triggers synaptic plasticity modifications by changing expression of plasticity related genes.
Regarding drug addiction, some epigenetic marks seem fundamental and upstream as illustrated by HDAC inhibitors effect on drug-induced synaptic and behavioural modifications [ 178 , 198 , 199 , 200 ]. Additionally, Maze et al. [ 201 ] demonstrated morphological plasticity induced by cocaine through the histone methyltransferase G9a. Again advocating for causal epigenetic, Authement et al. [ 66 ] demonstrated that HDAC inhibition locally in the VTA is sufficient to reverse epigenetic modifications and synaptic plasticity changes after morphine administration.
Two transcription factors implicated in addiction exemplify this bridging attempt: CREB and ∆FosB (a truncated form of the FosB gene) are both activated by several drugs of abuse [ 202 ]. CREB activation occurs in both subtypes of NAc MSNs (D1R and D2R), while ∆FosB activation is limited to D1R MSNs in response to all drugs of abuse except for opioids, which remarkably induce the protein in both MSNs [ 203 ]. Expression of active CREB in NAc MSNs increases their excitability [ 204 ] and underlies drug-induced long-term synaptic plasticity and associated changes in dendritic spine plasticity [ 205 ]. ∆FosB is also linked to synaptic plasticity but evokes contrasting effects on the two MSN subtypes, with increased AMPA receptor function induced in D1R MSNs and decreased AMPA receptor function induced in D2R MSNs [ 206 ]. Renthal et al. [ 150 ] unravelled CREB and ∆FosB target genes and observed that these genes are mainly involved in neuronal excitability and synaptic function. Moreover, as already briefly discussed above, CREB and ∆FosB action have also been related to multiple epigenetic regulations, including histone acetylation and methylation [ 150 ]. Besides, a novel mechanism for bridging the gap between epigenetic control of transcription and synapse plasticity might be seen in microRNAs [ 207 ]. The most studied miRNA in the context of synaptic plasticity is miR-132 and is known to be CREB-dependent [ 208 ]. In the striatum, miR-212 targets the epigenetic regulator methyl CpG binding protein 2 (MeCP2). MeCP2 acts as a transcriptional repressor through recruitment of histone deacetylases to methylated DNA segments [ 209 , 210 ].
Clinical treatments for drug addiction
Besides psychosocial interventions [ 211 ] such as cognitive behavioural therapy, the most widely used treatment for drug addiction involves agonist-like medication, a solution inadequately called replacement or substitution therapy [ 212 ]. This type of treatment has been successfully implemented in the daily practice for opioid use disorder (e.g.: methadone, buprenorphine) [ 213 ] and tobacco use disorder (e.g.: nicotine patch or gum, varenicline) [ 214 ]. Currently, this agonist-like treatment is also promising for psychostimulant use disorder [ 215 ]. Still, considering the addictive drug-like effect, the risk of abuse, misuse and diversion, replacement therapy should be prescribed with caution [ 215 , 216 ].
Recently, a randomised and control study on a cocaine vaccine failed to show an effectiveness but instead raised an important issue: immunised subjects may have increased their cocaine use to overcome the competitive anti-cocaine antibody inhibition [ 217 ]. Even though significant improvements have been developed for immunopharmacotherapies for psychostimulant addiction over the last decade, very few candidates have been evaluated so far in clinical trials [ 218 ]. These considerations are some of the reasons why other treatments for drug addiction should emerge with the help of neurobiological research [ 219 ].
Following successful subthalamic nucleus DBS for Parkinson’s disease [ 220 , 221 , 222 ], DBS was investigated for diverse psychiatric diseases including depression [ 223 ], obsessive-compulsive disorder [ 224 ] and Tourette syndrome [ 225 ]. Today, indications for DBS are enlarging, with several positive case reports and small cases series that studied NAc DBS for drug addiction. The first studies showing potential positive effects on drug addiction were reports on application of NAc DBS primarily intended for other medication-refractory neuropsychiatric disorder where a comorbid drug addiction was unexpectedly resolved [ 226 , 227 ]. For DBS treatment in drug addiction, it seems that clinical empirical results led to further bench investigations and refinement [ 88 , 103 , 228 , 229 , 230 , 231 ], or at least, clinical and animal studies evolved in parallel with poor connectivity between the two.
Afterwards, many case reports and small cases series studied NAc DBS being used primarily for drug addiction, all showing encouraging decreases in drug use [ 232 , 233 , 234 , 235 , 236 , 237 ]. However, these studies are limited by their descriptive nature, inconstant follow-up, multiple publication bias, small patient numbers and lack of blinded stimulation and standardised outcome measures. At this stage, additional preclinical and clinical research are needed to clarify the role of DBS in the treatment of drug addiction [ 237 ]. Currently, randomised and control clinical studies are conducted (NCT01245075).
In a recent review, Sanna et al. [ 238 ] highlighted how repetitive transcranial magnetic stimulation (rTMS) confirms the hypodopaminergic hypothesis of drug addiction. While enhancing dopaminergic function through direct or indirect pharmacological approaches does not significantly alleviate symptoms, in numerous studies, and has not yielded a single FDA-approved medication [ 239 ], rTMS might indirectly modulate the dopaminergic system. Many rTMS studies stimulate the dorsolateral PFC [ 240 , 241 ] that projects to the VTA and thus induces an increase in dopamine release in the synaptic cleft in the NAc [ 55 , 242 , 243 ]. Nevertheless, considering the heterogeneity of methods used in rTMS studies during the last 10 years [ 238 ], protocols and guidelines, were recently suggested by an international network of experts in neuromodulation and addiction to improve homogeneity of studies [ 244 ]. From this report, it is clear that multiple technical details for optimal stimulation need further investigations that might be achieved through preclinical studies. For example, low frequency (but not high-frequency) rTMS before methamphetamine exposure in rats blocked drug-induced conditioned place preference [ 245 ]. Being non-invasive, with insignificant side effects, rTMS could be seen as a great opportunity for drug addiction treatment. We are currently waiting for the results of a randomised and control study that aims at determine if, in heavy alcohol users, a single session of TMS can lower a patient’s craving and brain response to alcohol cues (NCT02939313).
Interesting views of clinical treatments for drug addiction are discussed in some other reviews [ 212 , 215 , 216 , 219 ]. The clinical impact of new treatments also depends on their translation into clinical practice which is mainly promoted by the pharmaceutical industry [ 219 ]. Indeed, even when an effective treatment is identified through basic research, it is commonly challenging to translate it to clinical practice, as illustrated by naltrexone as a treatment of alcoholism [ 219 ]. Another example of problematic translation to clinic is illustrated by modafinil, a treatment that has been reported to attenuate cocaine euphoria but for which larger clinical randomised and controlled studies showed controversial results [ 246 , 247 ].
Future directions
Drug addiction is a brain disease strongly influenced by environment and psychosocial aspects. The psychosocial conditions in which it has developed are extremely important. Exposure to conditioned cues can be a central issue in causing drug cravings and relapses, even after successful treatment, and thus they have to be minimised [ 2 , 74 , 77 ]. The pathophysiological aspects are particularly unsteady. For instance, as discussed in this review and in other ones [ 73 , 248 ], synaptic plasticity is dynamically altered after psychostimulant administration, so that a treatment could have opposite effects depending on timing aspects of the administration protocol. In addition, a prolonged treatment may involve compensatory mechanisms, giving unexpected results (e.g.: when HDAC inhibitors and psychostimulants are both administered acutely, they have synergistic effects through hyperacetylation and thus transcriptional activation of psychostimulant-regulated target genes. Conversely, when a drug of abuse is given in the context of chronic HDAC inhibitor, compensatory mechanisms may promote acetylated histone to the promoters of genes responsible for inducing histone methylation and thus chromatin condensation and gene repression, all of which, in turn, gave opposite effect [ 183 ]). Thus, the evolution through the different stages of the disease has to be taken into account [ 249 ] and treatment must follow them. These two aspects have to be incorporated in a holistic treatment strategy. Besides, studying combination of different cutting-edge approaches, with animal models of addiction, such as targeted rTMS or DBS with more systemic epigenetic modulation might show a better restoration of altered synaptic transmission and decrease the probability of relapse in drug addiction. Basically, drug addiction is a disease that seems to be difficult to treat preventively but it is more conceivable to help patients that would be in an abstinence stage not to experience relapse of their disease. As addiction is chronic and relapsing, a good treatment outcome is a significant reduction of drug administration and long periods of withdrawal, with only sporadic relapses [ 2 ].
It is clear that the main issues for optimal therapeutic management of this specific psychiatric disease belong to its dynamic complexity, diverse temporal evolution and undeniably psychosocial aspects. In this review, we focused mostly on the effects of drugs of abuse on synaptic plasticity and epigenetic modifications. Nowadays, these two subfields are mostly studied separately and the understanding of how these two main addictive drug-induced brain modifications interact might be fundamental for addiction research [ 6 ]. Indeed, argument for clinical trials for new treatments emerge from fundamental behavioural studies that should be implemented in a global approach to the addicted patient.
Conclusions
Here, we highlight, from a vast fundamental literature (mainly based on rodent models), promising therapeutics that would potentially treat drug addiction. Based on effect, on synaptic plasticity and epigenetic mechanisms, treatments such as GluA2-lacking AMPAR antagonists [ 72 , 84 ], mGluR1 positive allosteric modulator [ 85 ], NAc 12Hz-DBS [ 103 ] (in line with other promising neuromodulation therapeutics such as rTMS or transcranial direct current stimulation [ 250 ]), N-acetylcysteine [ 108 ], HDAC inhibitors [ 183 ] or even (in very early stages of investigation) CRISPR/dCas9 epigenetic editing [ 194 , 195 ] could be potential candidates for human randomised clinical trials (Fig. 3 ).
Finally, it is fundamental to consider the specific clinical aspects of the disease that would help to develop a personalised-treatment strategy. Indeed, after going from the bench to the bedside it will also be essential to assess the reversed route.
Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88.
Article Google Scholar
Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47.
Article CAS PubMed Google Scholar
Nielsen DA, Nielsen EM, Dasari T, Spellicy CJ. Pharmacogenetics of addiction therapy. Methods Mol Biol. 2014;1175:589–624.
Article PubMed CAS Google Scholar
Everitt BJ & Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2015;67:23–50.
Article PubMed Google Scholar
Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41.
Nestler EJ, Lüscher C. The molecular basis of drug addiction: linking epigenetic to synaptic and circuit mechanisms. Neuron. 2019;102:48–59.
Article CAS PubMed PubMed Central Google Scholar
Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431–43.
Article PubMed PubMed Central Google Scholar
Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–28.
Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–68.
Egervari G, Ciccocioppo R, Jentsch JD, Hurd YL. Shaping vulnerability to addiction – the contribution of behavior, neural circuits and molecular mechanisms. Neurosci Biobehav Rev. 2018;85:117–25.
Li MD, Burmeister M. New insights into the genetics of addiction. Nat. Rev. Genet. 2009;10:225–31.
Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7.
Shohat S, Amelan A, Shifman S. Convergence and divergence in the genetics of psychiatric disorders from pathways to developmental stages. Biol Psychiatry. 2021;89:32–40.
Kandel, DB. Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis (Cambridge University Press, 2002).
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32.
Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007;83:277–92.
Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66.
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8.
Tanda G, Pontieri FE, Chiara GD. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common µ1 opioid receptor mechanism. Science. 1997;276:2048–50.
Pontieri FE, Tanda G, Orzi F, Chiara GD. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–7.
Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9.
Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10.
Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–50.
Volkow ND, Fowler JS, Wang G-J, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–69.
Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–707.
Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J. Neurosci. 2002;22:8653–60.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38.
Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol. Psychiatry. 2016;79:39–46.
Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology. 2013;229:387–413.
Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature. 2018;564:366–71.
Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58.
Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–3.
Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–41.
Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86:1145–57.
Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2.
Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78.
Henny P, Brown MT, Northrop A, Faunes M, Ungless MA, Magill PJ, et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15:613–9.
Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55.
Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–68.
Edwards NJ, Tejeda HA, Pignatelli M, Zhang S, McDevitt RA, Wu J, et al. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat Neurosci. 2017;20:438–48.
Menegas W, Bergan JF, Ogawa SK, Isogai Y, Umadevi Venkataraju K, Osten P, et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife. 2015;4:e10032.
Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–4.
CAS PubMed Google Scholar
Young KA, Franklin TR, Roberts DC, Jagannathan K, Suh JJ, Wetherill RR, et al. Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J Neurosci. 2014;34:5038–43.
Article PubMed PubMed Central CAS Google Scholar
Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–70.
Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–23.
Slattery DA, Markou A, Froestl W, Cryan JF. The GABA B receptor-positive modulator GS39783 and the GABA B receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology. 2005;30:2065–72.
Hotsenpiller G, Wolf ME. Baclofen attenuates conditioned locomotion to cues associated with cocaine administration and stabilizes extracellular glutamate levels in rat nucleus accumbens. Neuroscience. 2003;118:123–34.
Brebner K, Phelan R, Roberts DCS. Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2000;66:857–62.
Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320:1517–21.
Sammaraiee Y, Yardley M, Keenan L, Buchanan K, Stevenson V, Farrell R. Intrathecal baclofen for multiple sclerosis related spasticity: a twenty year experience. Mult Scler Relat Disord. 2019;27:95–100.
Reynoard J, Schmitt C, Torrents R, Simon N. Toxicological considerations in the prescription of baclofen for the treatment of substance use disorders. Expert Opin Drug Metab Toxicol. 2020;16:309–17.
Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010;34:1849–57.
Yamini D, Lee SH, Avanesyan A, Walter M, Runyon B. Utilization of baclofen in maintenance of alcohol abstinence in patients with alcohol dependence and alcoholic hepatitis with or without cirrhosis. Alcohol Alcohol. 2014;49:453–6.
Addolorato G, Leggio L, Hopf FW, Diana M, Bonci A. Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology. 2012;37:163–77.
Kahn R, Biswas K, Childress AR, Shoptaw S, Fudala PJ, Gorgon L, et al. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend. 2009;103:59–64.
Dore GM, Lo K, Juckes L, Bezyan S, Latt N. Clinical experience with baclofen in the management of alcohol-dependent patients with psychiatric comorbidity: a selected case series. Alcohol Alcohol. 2011;46:714–20.
Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–90.
Liu Q, Pu L, Poo M. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–31.
Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858–71.
Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with γ-vinyl GABA. Synapse. 2003;50:261–5.
Barker JS, Hines RM. Regulation of GABAA receptor subunit expression in substance use disorders. Int J Mol Sci. 2020;21:4445.
Article CAS PubMed Central Google Scholar
Tan KR, Rudolph U, Lüscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34:188–97.
Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis. 2008;27:37–48.
Dacher M, Nugent FS. Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology. 2011;61:1166–71.
Authement ME, Langlois LD, Kassis H, Gouty S, Dacher M, Shepard RD, et al. Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition. J Neurophysiol. 2016;116:1093–103.
Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–30.
Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 2006;9:868–9.
Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–23.
Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–51.
Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–33.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. https://doi.org/10.1038/nature06995 .
Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure:when, how, and why? Front Mol Neurosci. 2012;5:72.
Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2.
Sadler-Smith E. Wallas’ four-stage model of the creative process: more than meets the eye? Creat Res J. 2015;27:342–52.
Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–13.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18.
Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry. 2016;73:1127–34.
Lippman-Bell JJ, Zhou C, Sun H, Feske JS, Jensen FE. Early-life seizures alter synaptic calcium-permeable AMPA receptor function and plasticity. Mol Cell Neurosci. 2016;76:11–20.
Achuta VS, Möykkynen T, Peteri UK, Turconi G, Rivera C, Keinänen K, et al. Functional changes of AMPA responses in human induced pluripotent stem cell-derived neural progenitors in fragile X syndrome. Sci Sÿignal. 2018;11:eaan8784.
Article CAS Google Scholar
Selvaraj BT, Livesey MR, Zhao C, Gregory JM, James OT, Cleary EM, et al. C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca 2+ -permeable AMPA receptor-mediated excitotoxicity. Nat Commun. 2018;9:347.
Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P. Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson’s disease. J Neurochem. 2010;114:499–511.
Whitehead G, Regan P, Whitcomb DJ, Cho K. Ca2+-permeable AMPA receptor: a new perspective on amyloid-beta mediated pathophysiology of Alzheimer’s disease. Neuropharmacology. 2017;112:221–7.
Twomey EC, Yelshanskaya MV, Vassilevski AA, Sobolevsky AI. Mechanisms of channel block in calcium-permeable AMPA receptors. Neuron. 2018;99:956–.e4.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat. Neurosci. 2014;17:73–80.
Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol. Psychiatry. 2016;80:661–70.
Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–64.
Ma T, Cheng Y, Roltsch Hellard E, Wang X, Lu J, Gao X, et al. Bidirectional and long-lasting control of alcohol-seeking behavior by corticostriatal LTP and LTD. Nat. Neurosci. 2018;21:373–83.
Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75.
Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 2010;35:401–15.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91.
Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57:543–50.
Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–62.
De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–71.
Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–59.
Vorspan F, de Witt P, Zerdazi EH, Karsinti E, Ksouda K, Icick R, et al. Chronic exposure to cocaine is associated with persistent behavioral disturbances. A cross-sectional dimensional study in outpatients with multiple substance use disorders. Psychopharmacology. 2020;237:3399–407.
Vorspan F, Icick R, Mekdad N, Courtin C, Bloch V, Bellivier F, et al. Translational study of the whole transcriptome in rats and genetic polymorphisms in humans identifies LRP1B and VPS13A as key genes involved in tolerance to cocaine-induced motor disturbances. Transl Psychiatry. 2020;10:381.
Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120.
Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56:160–8.
Tzschentke TM. REVIEW ON CPP: measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462.
Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43.
Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–4.
Creed M, Pascoli VJ, Lüscher C. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–64.
Shen Y, Campbell RE, Côté DC, Paquet M-E. Challenges for therapeutic applications of opsin-based optogenetic tools in humans. Front Neural Circuits. 2020;14:41.
Vorspan F, Mallet L, Corvol J-C, Pelissolo A, Lépine J-P. Treating addictions with deep brain stimulation is premature but well-controlled clinical trials should be performed. Addict Abingdon Engl. 2011;106:1535–6.
Mahoney JJ, Hanlon CA, Marshalek PJ, Rezai AR, Krinke L. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: review of modalities and implications for treatment. J Neurol Sci. 2020;418:117149.
Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51.
Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N -Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–9.
Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–9.
Vicente AM, Galvão-Ferreira P, Tecuapetla F, Costa RM. Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr Biol. 2016;26:R267–269.
Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–9.
Durieux PF, Schiffmann SN, de Kerchove d’Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012;31:640–53.
Cox J, Witten IB. Striatal circuits for reward learning and decision-making. Nat Rev Neurosci. 2019;20:482–94.
Lalchandani RR, van der Goes M-S, Partridge JG, Vicini S. Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J Neurosci. 2013;33:14075–86.
Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron. 2016;90:1100–13.
Dobbs LK, Kaplan AR, Bock R, Phamluong K, Shin JH, Bocarsly ME, et al. D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology. 2019;44:805–16.
Tecuapetla F, Koós T, Tepper JM, Kabbani N, Yeckel MF. Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci. 2009;29:8977–90.
Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the fourth dimension regulate drug relapse. Trends Neurosci. 2016;39:472–85.
Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–7.
Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci USA. 2011;108:385–90.
Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–84.
Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84.
Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 2012;13:743–57.
Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–9.
Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, et al. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102:1548–60.
Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS ONE. 2012;7:e37541.
Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Chronic intermittent ethanol exposure enhances the excitability and synaptic plasticity of lateral orbitofrontal cortex neurons and induces a tolerance to the acute inhibitory actions of ethanol. Neuropsychopharmacology. 2016;41:1112–27.
Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–37.
Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stählin O, Heilig M, et al. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–806.
Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–8.
Baptista MAS, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–7.
Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–9.
Caprioli D, Justinova Z, Venniro M, Shaham Y. Effect of novel allosteric modulators of metabotropic glutamate receptors on drug self-administration and relapse: a review of preclinical studies and their clinical implications. Biol Psychiatry. 2018;84:180–92.
Clark AM et al. Dopamine D2 receptors in the paraventricular thalamus attenuate cocaine locomotor sensitization. eNeuro. 2017;4:ENEURO.0227-17.2017.
Dong X, Li S, Kirouac GJ. Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct. Funct. 2017;222:3927–43.
Huang AS, Mitchell JA, Haber SN, Alia-Klein N & Goldstein RZ. The thalamus in drug addiction: from rodents to humans. Philos Trans R Soc Lond B Biol Sci. 2018;373:20170028.
Perez SM, Lodge DJ. Convergent inputs from the hippocampus and thalamus to the nucleus accumbens regulate dopamine neuron activity. J Neurosci. 2018;38:10607–18.
Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic–thalamic–striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85.
Penzo MA & Gao C. The paraventricular nucleus of the thalamus: an integrative node underlying homeostatic behavior. Trends Neurosci. 2021. https://doi.org/10.1016/j.tins.2021.03.001 .
De Groote A & de Kerchove d'Exaerde A. Thalamo-Nucleus Accumbens projections in motivated behaviors and addiction. Front Syst Neurosci. 2021;15:711350.
Zhu Y, Wienecke CFR, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–22.
Chisholm A, Rizzo D, Fortin É, Moman V, Quteishat N, Romano A, et al. Assessing the role of corticothalamic and thalamo-accumbens projections in the augmentation of heroin seeking in chronically food-restricted rats. J Neurosci. 2021;41:354–65.
Otis JM, Zhu M, Namboodiri V, Cook CA, Kosyk O, Matan AM, et al. Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron. 2019;103:423–.e4.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12.
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–5.
Yang H-J, Zhang HY, Bi GH, He Y, Gao JT, Xi ZX. Deletion of type 2 metabotropic glutamate receptor decreases sensitivity to cocaine reward in rats. Cell Rep. 2017;20:319–32.
Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4.
De Backer J-F, et al. Deletion of Maged1 in mice abolishes locomotor and reinforcing effects of cocaine. EMBO Rep. 2018;19:e45089.
Karadurmus D, Rial D, De Backer JF, Communi D, de Kerchove d'Exaerde A, Schiffmann SN. GPRIN3 controls neuronal excitability, morphology, and striatal-dependent behaviors in the indirect pathway of the striatum. J. Neurosci. 2019;39:7513–28.
Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, Maze I, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–48.
Ena SL, De Backer J-F, Schiffmann SN, de Kerchove d’Exaerde A. FACS array profiling identifies Ecto-5’ nucleotidase as a striatopallidal neuron-specific gene involved in striatal-dependent learning. J Neurosci. 2013;33:8794–809.
Iida, N & Kozasa, T. 29 - Identification and biochemical analysis of GRIN1 and GRIN2. In: Siderovski DP, editor. Methods in Enzymology. Academic Press; 2004;390:475–83.
Mototani Y, Okamura T, Goto M, Shimizu Y, Yanobu-Takanashi R, Ito A, et al. Role of G protein-regulated inducer of neurite outgrowth 3 (GRIN3) in β-arrestin 2-Akt signaling and dopaminergic behaviors. Pflügers Arch. 2018;470:937–47.
Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–5.
David Smith A, Paul Bolam J. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–65.
Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–604.
Spencer S, Garcia-Keller C, Roberts-Wolfe D, Heinsbroek JA, Mulvaney M, Sorrell A, et al. Cocaine use reverses striatal plasticity produced during cocaine seeking. Biol Psychiatry. 2017;81:616–24.
MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type– and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17:1198–207.
Dos Santos M, Cahill EN, Bo GD, Vanhoutte P, Caboche J, Giros B, et al. Cocaine increases dopaminergic connectivity in the nucleus accumbens. Brain Struct Funct. 2018;223:913–23.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54.
Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76:259–68.
Hamilton PJ, Nestler EJ. Epigenetics and addiction. Curr Opin Neurobiol. 2019;59:128–36.
Handel AE, Ramagopalan SV. Is Lamarckian evolution relevant to medicine? BMC Med Genet. 2010;11:73.
Jablonka E & Lamb MJ. Epigenetic Inheritance And Evolution: The Lamarckian Dimension. Oxford University Press; 1999.
Springer JT & Holley D. An Introduction To Zoology: Investigating The Animal World. Jones & Bartlett Learning; 2013.
Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 2013;16:42–47.
Goldberg LR, Gould TJ. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur J Neurosci. 2019;50:2453–66.
Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–44.
Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120:202–9.
Gavin DP, et al. Stable histone methylation changes at proteoglycan network genes following ethanol exposure. Front Genet. 2018;9:346.
Sadakierska-Chudy A, Frankowska M, Jastrzębska J, Wydra K, Miszkiel J, Sanak M, et al. Cocaine administration and its withdrawal enhance the expression of genes encoding histone-modifying enzymes and histone acetylation in the rat prefrontal cortex. Neurotox Res. 2017;32:1–10.
Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17:487–500.
Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, et al. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIα in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35:913–28.
Brami-Cherrier K. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–54.
Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–7.
Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors. J Neurosci. 2011;31:16941–8.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25.
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14.
Sun J, Wang L, Jiang B, Hui B, Lv Z, Ma L. The effects of sodium butyrate, an inhibitor of histone deacetylase, on the cocaine- and sucrose-maintained self-administration in rats. Neurosci Lett. 2008;441:72–76.
Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J. Neurosci. 2008;28:9342–8.
Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci USA. 2005;102:19186–91.
Kalda A, Heidmets L-T, Shen H-Y, Zharkovsky A, Chen J-F. Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav. Brain Res. 2007;181:76–84.
Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16:434–40.
LaPlant Q, Vialou V, Covington HE, Dumitriu D, Feng J, Warren BL, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–43.
Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, et al. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 2015;35:8042–58.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–85.
Brami‐Cherrier K, Roze E, Girault J-A, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108:1323–35.
Scobie KN, Damez-Werno D, Sun H, Shao N, Gancarz A, Panganiban CH, et al. Essential role of poly(ADP-ribosyl)ation in cocaine action. Proc Natl Acad Sci USA. 2014;111:2005–10.
Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, et al. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science. 2020;368:197–201.
Sun H, Maze I, Dietz DM, Scobie KN, Kennedy PJ, Damez-Werno D, et al. Morphine epigenomically regulates behavior through alterations in histone H3 lysine 9 dimethylation in the nucleus accumbens. J Neurosci. 2012;32:17454–64.
Maze I, Feng J, Wilkinson MB, Sun H, Shen L, Nestler EJ. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proc Natl Acad Sci USA. 2011;108:3035–40.
Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, et al. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–247.e17.
Liao H-K, Hatanaka F, Araoka T, Reddy P, Wu MZ, Sui Y, et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell. 2017;171:1495–1507.e15.
Heller EA, Cates HM, Peña CJ, Sun H, Shao N, Feng J, et al. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat. Neurosci. 2014;17:1720–7.
Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Peña CJ, Neve RL, et al. Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. J Neurosci. 2016;36:4690–7.
Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS ONE. 2011;6:e19958.
Massart R, Freyburger M, Suderman M, Paquet J, El Helou J, Belanger-Nelson E, et al. The genome-wide landscape of DNA methylation and hydroxymethylation in response to sleep deprivation impacts on synaptic plasticity genes. Transl Psychiatry. 2014;4:e347–e347.
Kyzar EJ, Pandey SC. Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett. 2015;601:11–19.
Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 2013;38:94–110.
Kyzar EJ, Floreani C, Teppen TL & Pandey SC. Adolescent alcohol exposure: burden of epigenetic reprogramming, synaptic remodeling, and adult psychopathology. Front. Neurosci. 2016;10:222.
Maze I, Covington HE, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–6.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37.
Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–95.
Huang YH, Lin Y, Brown TE, Han MH, Saal DB, Neve RL, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–7.
Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA. 1997;94:1482–7.
Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110:1923–8.
Smith ACW, Kenny PJ. MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. 2018;17:e12424.
Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, et al. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–8.
CAS PubMed PubMed Central Google Scholar
Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci USA. 2007;104:19416–21.
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–9.
De Crescenzo F, Ciabattini M, D'Alò GL, De Giorgi R, Del Giovane C, Cassar C, et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLOS Med. 2018;15:e1002715.
Phillips KA, Epstein DH, Preston KL. Psychostimulant addiction treatment. Neuropharmacology. 2014;87:150–60.
Mattick RP, Breen C, Kimber J & Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009;CD002209. https://doi.org/10.1002/14651858.CD002209.pub2 .
Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C & Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD000146.pub5 .
Tardelli VS, Bisaga A, Arcadepani FB, Gerra G, Levin FR, Fidalgo TM. Prescription psychostimulants for the treatment of stimulant use disorder: a systematic review and meta-analysis. Psychopharmacology. 2020;237:2233–55.
Darke S & Farrell M. Which medications are suitable for agonist drug maintenance? Addiction. 2016;111:767–74.
Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42–47.
Hossain MK, Hassanzadeganroudsari M, Nurgali K, Apostolopoulos V. Vaccine development against methamphetamine drug addiction. Expert Rev Vaccines. 2020;19:1105–14.
Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–6.
Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–6.
Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–8.
Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, et al. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95.
Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;1991:1374–83.
Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–93.
Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–80.
Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78:1152–3.
Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66:E218.
Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–89.
Liu H-Y, Jin J, Tang JS, Sun WX, Jia H, Yang XP, et al. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13:40–46.
Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–9.
Batra V, Tran TL, Caputo J, Guerin GF, Goeders NE, Wilden J. Intermittent bilateral deep brain stimulation of the nucleus accumbens shell reduces intravenous methamphetamine intake and seeking in Wistar rats. J Neurosurg. 2017;126:1339–50.
Heinze H-J, et al. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci. 2009;3:22.
Kuhn J, Gründler TO, Bauer R, Huff W, Fischer AG, Lenartz D, et al. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol. 2011;16:620–3.
Kuhn J, Möller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TO, et al. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry. 2014;19:145–6.
Valencia-Alfonso C-E, Luigjes J, Smolders R, Cohen MX, Levar N, Mazaheri A, et al. Effective deep brain stimulation in heroin addiction: a case report with complementary intracranial electroencephalogram. Biol Psychiatry. 2012;71:e35–37.
Voges J, Müller U, Bogerts B, Münte T, Heinze H-J. Deep brain stimulation surgery for alcohol addiction. World Neurosurg. 2013;80:S28.e21–31.
Wang TR, Moosa S, Dallapiazza RF, Elias WJ, Lynch WJ. Deep brain stimulation for the treatment of drug addiction. Neurosurg Focus. 2018;45:E11.
Sanna A, Fattore L, Badas P, Corona G, Diana M. The hypodopaminergic state ten years after: transcranial magnetic stimulation as a tool to test the dopamine hypothesis of drug addiction. Curr Opin Pharmacol. 2021;56:61–67.
Verrico CD, Haile CN, Newton TF, Kosten TR, Garza RDL. Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs. 2013;22:1549–68.
Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–60.
Pettorruso M, Di Giuda D, Martinotti G, Cocciolillo F, De Risio L, Montemitro C, et al. Dopaminergic and clinical correlates of high-frequency repetitive transcranial magnetic stimulation in gambling addiction: a SPECT case study. Addict Behav. 2019;93:246–9.
Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE. 2009;4:e6725.
Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–15.
Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev. 2019;104:118–40.
Wu X-Q, Zan GY, Ju YY, Chen TZ, Guo LB, Jiao DL, et al. Low-frequency repetitive transcranial magnetic stimulation inhibits the development of methamphetamine-induced conditioned place preference. Behav Brain Res. 2018;353:129–36.
Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abus Treat. 2012;43:303–12.
Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–33.
Chiamulera C, Piva A, Abraham WC. Glutamate receptors and metaplasticity in addiction. Curr Opin Pharmacol. 2021;56:39–45.
Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–93.
Luigjes J, Segrave R, de Joode N, Figee M, Denys D. Efficacy of invasive and non-invasive brain modulation interventions for addiction. Neuropsychol Rev. 2019;29:116–38.
Jordi E, Heiman M, Marion-Poll L, Guermonprez P, Cheng SK, Nairn AC, Greengard P, Girault J-A. Proceedings of the National Academy of Sciences . 2013;110:9511-16. https://doi.org/10.1073/pnas.1307116110 .
Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–8.
Download references
Acknowledgements
We thank Michele Zoli, Romain Icick and Daniel Rial for helpful comments and corrections on the manuscript. Julian Cheron is supported by a fellowship of the FRS-FNRS (Belgium). Alban de Kerchove d´Exaerde is a Research Director of the FRS-FNRS. FRS-FNRS (Belgium). Fondation Simone et Pierre Clerdent, Fondation ULB, supported this study.
Author information
Authors and affiliations.
Laboratory of Neurophysiology, ULB Neuroscience Institute, Université Libre de Bruxelles (ULB), Brussels, B-1070, Belgium
Julian Cheron & Alban de Kerchove d’Exaerde
You can also search for this author in PubMed Google Scholar
Contributions
JC and AKE wrote the paper and AKE supervised all the work.
Corresponding author
Correspondence to Alban de Kerchove d’Exaerde .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cheron, J., Kerchove d’Exaerde, A.d. Drug addiction: from bench to bedside. Transl Psychiatry 11 , 424 (2021). https://doi.org/10.1038/s41398-021-01542-0
Download citation
Received : 05 April 2021
Revised : 14 July 2021
Accepted : 23 July 2021
Published : 12 August 2021
DOI : https://doi.org/10.1038/s41398-021-01542-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Snp-based and haplotype-based genome-wide association on drug dependence in han chinese.
- Yangyang Liu
BMC Genomics (2024)
Unraveling Relapse in Male Forensic Psychiatric Patients with Substance Use Disorders—The Impact of Social, Psychiatric, and Personality Factors Post Long-Term Remission
- Michael Fritz
- Felipe Montiel
- Judith Streb
International Journal of Mental Health and Addiction (2024)

USP7/Maged1-mediated H2A monoubiquitination in the paraventricular thalamus: an epigenetic mechanism involved in cocaine use disorder
- Julian Cheron
- Leonardo Beccari
- Alban de Kerchove d’Exaerde
Nature Communications (2023)
Methylation and expression quantitative trait locus rs6296 in the HTR1B gene is associated with susceptibility to opioid use disorder
- Jianbo Zhang
Psychopharmacology (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Signs of Addiction
Addiction Research
Discover the latest in addiction research, from the neuroscience of substance use disorders to evidence-based treatment practices. reports, updates, case studies and white papers are available to you at hazelden betty ford’s butler center for research..

Why do people become addicted to alcohol and other drugs? How effective is addiction treatment? What makes certain substances so addictive? The Butler Center for Research at the Hazelden Betty Ford Foundation investigates these and other questions and publishes its scientific findings in a variety of alcohol and drug addiction research papers and reports. Research topics include:
- Evidence-based treatment practices
- Addiction treatment outcomes
- Addiction, psychiatry and the brain
- Addictive substances such as prescription opioids and heroin
- Substance abuse in youth/teens, older adults and other demographic groups such as health care or legal professionals
These research queries and findings are presented in the form of updates, white papers and case studies. In addition, the Butler Center for Research collaborates with the Recovery Advocacy team to study special-focus addiction research topics, summarized in monthly Emerging Drug Trends reports. Altogether, these studies provide the latest in addiction research for anyone interested in learning more about the neuroscience of addiction and how addiction affects individuals, families and society in general. The research also helps clinicians and health care professionals further understand, diagnose and treat drug and alcohol addiction. Learn more about each of the Butler Center's addiction research studies below.
Research Updates
Written by Butler Center for Research staff, our one-page, topic-specific summaries discuss current research on topics of interest within the drug abuse and addiction treatment field.
View our most recent updates, or view the archive at the bottom of the page.
Patient Outcomes Study Results at Hazelden Betty Ford
Trends and Patterns in Cannabis Use across Different Age Groups
Alcohol and Tobacco Harm Reduction Interventions
Harm Reduction: History and Context
Racial and Ethnic Health Disparities and Addiction
Psychedelics as Therapeutic Treatment
Sexual and Gender Minority Youth and SUDs
Health Care Professionals and Mental Health
Grief and Addiction
Helping Families Cope with Addiction
Emerging Drug Trends Report and National Surveys
Shedding New Light on America’s No. 1 Health Problem
In collaboration with the University of Maryland School of Public Health and with support from the Butler Center for Research, the Recovery Advocacy team routinely issues research reports on emerging drug trends in America. Recovery Advocacy also commissions national surveys on attitudes, behaviors and perspectives related to substance use. From binge drinking and excessive alcohol use on college campuses, to marijuana potency concerns in an age of legalized marijuana, deeper analysis and understanding of emerging drug trends allows for greater opportunities to educate, inform and prevent misuse and deaths.
Each drug trends report explores the topic at hand, documenting the prevalence of the problem, relevant demographics, prevention and treatment options available, as well as providing insight and perspectives from thought leaders throughout the Hazelden Betty Ford Foundation.
View the latest Emerging Drug Trends Report:
Pediatricians First Responders for Preventing Substance Use
- Clearing Away the Confusion: Marijuana Is Not a Public Health Solution to the Opioid Crisis
- Does Socioeconomic Advantage Lessen the Risk of Adolescent Substance Use?
- The Collegiate Recovery Movement Is Gaining Strength
- Considerations for Policymakers Regarding Involuntary Commitment for Substance Use Disorders
- Widening the Lens on the Opioid Crisis
- Concerns Rising Over High-Potency Marijuana Use
- Beyond Binging: “High-Intensity Drinking”
View the latest National Surveys :
- College Administrators See Problems As More Students View Marijuana As Safe
College Parents See Serious Problems From Campus Alcohol Use
- Youth Opioid Study: Attitudes and Usage
About Recovery Advocacy
Our mission is to provide a trusted national voice on all issues related to addiction prevention, treatment and recovery, and to facilitate conversation among those in recovery, those still suffering and society at large. We are committed to smashing stigma, shaping public policy and educating people everywhere about the problems of addiction and the promise of recovery. Learn more about recovery advocacy and how you can make a difference.
Evidence-Based Treatment Series
To help get consumers and clinicians on the same page, the Butler Center for Research has created a series of informational summaries describing:
- Evidence-based addiction treatment modalities
- Distinctive levels of substance use disorder treatment
- Specialized drug and alcohol treatment programs
Each evidence-based treatment series summary includes:
- A definition of the therapeutic approach, level of care or specialized program
- A discussion of applicability, usage and practice
- A description of outcomes and efficacy
- Research citations and related resources for more information
View the latest in this series:
Motivational Interviewing
Cognitive Behavioral Therapy
Case Studies and White Papers
Written by Hazelden Betty Ford Foundation researchers and clinicians, case studies and white papers presented by the Butler Center for Research provide invaluable insight into clinical processes and complex issues related to addiction prevention, treatment and recovery. These in-depth reports examine and chronicle clinical activities, initiatives and developments as a means of informing practitioners and continually improving the quality and delivery of substance use disorder services and related resources and initiatives.
- What does it really mean to be providing medication-assisted treatment for opioid addiction?
Adolescent Motivational Interviewing
Peer Recovery Support: Walking the Path Together
Addiction and Violence During COVID-19
The Brain Disease Model of Addiction
Healthcare Professionals and Compassion Fatigue
Moving to Trauma-Responsive Care
Virtual Intensive Outpatient Outcomes: Preliminary Findings
Driving Under the Influence of Cannabis
Vaping and E-Cigarettes
Using Telehealth for Addiction Treatment
Grandparents Raising Grandchildren
Substance Use Disorders Among Military Populations
Co-Occurring Mental Health and Substance Use Disorders
Women and Alcohol
Prescription Rates of Opioid Analgesics in Medical Treatment Settings
Applications of Positive Psychology to Substance Use Disorder
Substance Use Disorders Among Legal Professionals
Factors Impacting Early Alcohol and Drug Use Among Youths
Animal-Assisted Therapy for Substance Use Disorders
Prevalence of Adolescent Substance Misuse
Problem Drinking Behaviors Among College Students
The Importance of Recovery Management
Substance Use Factors Among LGBTQ individuals
Prescription Opioids and Dependence
Alcohol Abuse Among Law Enforcement Officers
Helping Families Cope with Substance Dependence
The Social Norms Approach to Student Substance Abuse Prevention
Drug Abuse, Dopamine and the Brain's Reward System
Women and Substance Abuse
Substance Use in the Workplace
Health Care Professionals: Addiction and Treatment
Cognitive Improvement and Alcohol Recovery
Drug Use, Misuse and Dependence Among Older Adults
Emerging Drug Trends
Does Socioeconomic Advantage Lessen the Risk of Adolescent Substance Use
The Collegiate Recovery Movement is Gaining Strength
Involuntary Commitment for Substance Use Disorders
Widening the Lens of the Opioid Crisis
Beyond Binge Drinking: High Intensity Drinking
High Potency Marijuana
National Surveys
College Administrators See Problems as More Students View Marijuana as Safe
Risky Opioid Use Among College-Age Youth
Case Studies/ White Papers
What does it really mean to be providing medication-assisted treatment for opioid addiction
Are you or a loved one struggling with alcohol or other drugs? Call today to speak confidentially with a recovery expert. Most insurance accepted.
Harnessing science, love and the wisdom of lived experience, we are a force of healing and hope for individuals, families and communities affected by substance use and mental health conditions..
We use our own and third-party cookies to improve your experience and to analyze the use of our website. Find out more about how we use cookies in our Privacy Policy .
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Understanding the Global Problem of Drug Addiction is a Challenge for IDARS Scientists
- Author information
- Article notes
- Copyright and License information
Address correspondence to this author at the Neurochemistry Laboratory, NCTR/FDA, Jefferson, AR, USA; Tel: +1 870-543-7123; E-mail: [email protected]
Received 2009 Oct 1; Revised 2010 Apr 17; Accepted 2010 May 26.
This is an open access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.5/ ), which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
IDARS is an acronym for the International Drug Abuse Research Society. Apart from our scientific and educational purposes, we communicate information to the general and scientific community about substance abuse and addiction science and treatment potential. Members of IDARS are research scientists and clinicians from around the world, with scheduled meetings across the globe. IDARS is developing a vibrant and exciting international mechanism not only for scientific interactions in the domain of addiction between countries but also ultimately as a resource for informing public policy across nations. Nonetheless, a lot more research needs to be done to better understand the neurobiological basis of drug addiction – A challenge for IDARS scientists.
Keywords: International Drug Abuse Research Society, IDARS, addiction, alcohol, marijuana, psychostimulants.
INTRODUCTION
Drug addiction is a chronically relapsing disorder that has been characterized by the compulsive use of addictive substances despite adverse consequences to the individual and society [ 1 ]. Addiction to drugs and alcohol is increasingly becoming a worldwide trend in lifestyle that is prevalent in rich and poor countries alike. Addiction to alcohol, drugs and cigarette smoking is now regarded as a major public health problem. Other forms of addiction including computer games, gambling, sex and food also have severe consequences on the health of the individual and to society. The commonly abused drugs have profound action in the nervous system, particularly in the brain. Some of these substances such as opium, marijuana, cocaine, nicotine, caffeine, mescaline, and psilocybin are obtained from natural sources while others are synthetic or designer drugs. Furthermore some of these substances like alcohol and nicotine are legal while some others that are legally available by prescription have addictive potential in vulnerable individuals. A number of addictive substances are illegal in most countries and this fuel the illegal drug trafficking and business that are often associated with criminal activities. The initiation of the use of these substances induces euphoria, reward and a state of well-being that can lead to physical and psychological dependences. Withdrawal syndrome occurs when the individual attempts to stop the use of addictive substances and this leads to the cycle of dependency. The mechanism(s) associated with the cycle of addiction include neuronal adaptation with tolerance or sensitization involved in the action of addictive substances. A number of factors have also been associated with addiction, including the availability, cost, method of administration, environmental factors such as behaviors acceptable in a community, peer influences and genetic and epigenetic factors. Over the years a number of therapeutic approaches for drug and alcohol addiction have been utilized. However, relapse the resumption of drug taking following a period of drug abstinence, is considered the main hurdle in treating drug addiction. Unfortunately pharmacological treatment of drug and alcohol dependency has largely been disappointing and new therapeutic targets and hypotheses are needed. Drug addiction is also influenced by the interaction of genes, epigenetics and the environment. Twin studies consistently show that there is a heritable component to drug abuse and addiction [ 2 ]. Now using modern genomic techniques, we are able to examine genetic variants, or single nucleotide polymorphisms that contribute to addiction vulnerability. So a lot more research needs to be done to better understand the neurobiological basis of drug addiction and hence a continuous challenge for IDARS scientists. IDARS is therefore engaged in a vibrant and exciting international mechanism, not only for scientific interactions among scientists in the domain of addiction research between countries but also as a resource for informing public policy across nations. This is an exciting period in the study of the neurobiology of addiction where brain circuitry and molecular mechanisms are providing hope for understanding not only the vulnerability to addiction but also providing new targets for the treatment of various types of substance abuse/dependence as presented in this report.
MARIJUANA HIGHLIGHTS
Various forms of marijuana preparations comes from the cannabis plant and is the most commonly used drug in the world, for recreation and suddenly, we are awakened to potential therapeutic applications. Therefore, these are high times for marijuana research with new findings on the biological effects of cannabinoids and as new potential applications in neurological and neuropsychiatric disorders [ 3 ]. The new advances and understanding indicate that the cellular, molecular and behavioral responses to marijuana are encoded in our genes [ 3 ]. The discovery that specific genes codes for cannabinoid receptors (CBRs) that are activated by marijuana use, and that the human body makes its own marijuana-like substances - endocannabinoids [ 4 ], that also activates CBRs have provided surprising new knowledge about cannabinoid genomic and proteomic profiles. These remarkable advances in understanding the biological actions of marijuana, cannabinoids and endocannabinoids, is unraveling the genetic basis of marijuana use and the implication in human health and disease. We know that the two well characterized cannabinoid CB1 and CB2 receptors are encoded by CNR1 and CNR2 genes that have been mapped to human chromosome 6 and 1 respectively. A number of variations in cannabinoid receptor genes have been associated with human disorders including drug dependency [ 4 ], osteoporosis [ 5 ], ADHD and PTSD, [ 6 , 7 ], obesity [ 8 , 9 ], and depression [ 10 , 11 ]. Thus, because of the ubiquitous distribution and role of the endocannabinoid system in the regulation of a variety of normal human physiology, drugs that are targeted to different aspects of this system are already benefiting cancer subjects and those with AIDs and metabolic syndromes [ 8 ]. In the coming era of personalized medicine, genetic variants and haplotypes in CNR1 and CNR2 genes associated with obesity or addiction phenotypes may help identify specific targets in conditions of endocannabinoid dysfunction. Most strikingly, variants of CNR genes co-occur with other genetic variations and share biological susceptibility that underlies comorbidity in many neuropsychiatric disturbances [ 12 ]. Therefore, understanding the endocannabinoid system in the human body and brain will contribute to elucidating this natural regulatory mechanism in health and disease.
CHRONIC ALCOHOLISM IN HUMAN SUBJECTS
Illicit drug use has well-known detrimental effects, but the legal drugs tobacco and alcohol have far greater impact on human health world-wide. By a standard measure of morbidity, dalys, tobacco is at 4.1%, alcohol 4.0% (6.6% for males, 3.1% for females [ 13 ]); all illicit drugs combined add only 0.8% to global disease burden [ 14 ]. Alcohol consumption is common: in one survey, 82% of respondents over 14 had imbibed it in the previous 12 months [ 15 ]. The social cost of alcohol use is also very high [ 16 , 17 ]. Alcohol misuse leads to selective brain pathology, though subjects differ markedly [ 18 ]. Brain shrinkage, reduced white-matter volume, and dendritic pruning may be reversible with abstinence; irreversible effects are more focal: superior prefrontal cortex (sfc) is particularly vulnerable [ 19 , 20 ]. Common comorbidities (cirrhosis of the liver and the Wernicke-Korsakoff syndrome), which are more prevalent with higher rates of alcohol consumption [ 21 ], lead to greater brain atrophy [ 18 ]. Neuronal death may be due to excitotoxicity , an imbalance between excitatory and inhibitory inputs mediated by NMDA receptors (NMDAR) and reduced GABA A -mediated responses [ 22 , 23 ]. This allostasis [ 24 , 25 ] maintains a proper balance between excitation and inhibition in the presence of alcohol. When alcohol is removed, as in periods of abstinence, the balance is skewed towards over-excitation. This leads to a large influx of Ca 2+ ions into cells, affecting many signaling pathways and eventually leading to cell death. In rat and mouse models NMDAR are increased after chronic alcohol, but there are regional and species differences due to modes of administration, age, time of withdrawal, etc [ 26 - 29 ]. All studies show increases in at least one NMDAR subunit [ 30 - 34 ]. However, the area affected and the subunit(s) that change are paradigm-dependent [ 30 , 32 ].
In human autopsy studies, NMDAR are increased in sfc and hippocampus [ 35 , 36 ]. Alcoholics without comorbid disease shift the excitatory balance by GABA A receptor subunit switching to reduce inhibitory function. GABA A shifts are small in cirrhotic alcoholics, and the key change may be increased excitability via altered NMDAR expression. Using quantitative real-time PCR we found that alcoholics without liver cirrhosis did not differ significantly from controls in the expression of any subunit, whereas all subunits were significantly lower in cirrhotic alcoholics [ 37 - 40 ]. Promising NMDAR-specific tracers for PET and SPECT provide the potential to study NMDAR changes in human subjects in the future [ 41 - 43 ].
Chronic alcohol misuse affects the expression of many genes in the brain, leading to long-term changes in neural function. Microarray and proteomic studies have found changes in the expression of genes involved in metabolism, immune response, cell survival, cell communication, signal transduction, and energy production, DNA-binding proteins, transcription factors, repair enzymes, myelination, and cell-adhesion [ 44 - 46 ].
The Genetics of Alcoholism
Alcoholism in human subjects is mediated by many societal and genetic factors. Genes may mediate etiology and pathogenesis, although this issue is still hotly debated. Different genetic markers are associated with increased risk of alcohol misuse, dependence, craving, tolerance, and withdrawal severity [ 47 ]. Risk-factor genes code for alcohol-metabolising enzymes, and also for the effectors of neurotransmission – receptors, transporters and signal-transduction components – for a variety of transmitter classes, including dopamine, serotonin, glutamate, and GABA. Some genes are associated with general aspects of addiction [ 48 , 49 ].
The effects of these polymorphisms can be divided into two categories: those that pre-dispose the individual to alcohol abuse, and those that make an individual more susceptible to the toxic effects of alcohol. Polymorphisms may not only alter the product of the parent gene (e.g., by changing the amino-acid codon) but may also have pleiotropic effects, i.e., the changes in one gene may affect the expression of, or activity of the product of, another gene. An emerging microarray literature is showing that knocking out a single gene alters the expression of hundreds of transcripts, many of which have no discernible association with the knocked-out gene. Knockout mice strains that differ in alcohol responsiveness have been compared to find transcripts that show discriminant expression [ 50 ]. Affected transcripts are from genes located on a range of chromosomes – not only that bearing the knocked-out gene. It is difficult to transfer this concept to human brain but an analogue of a gene knockout relevant to human alcoholism is the ALDH2 gene ( ALDH2 -2,2 homozygotes have no ALDH activity [ 51 ]). More generally, allelic variants of alcoholism-associated genes are very likely to moderate the expression of a range of genes. There is a multiplier effect for expression whereby proteins usually show larger effect sizes than mRNA transcripts [ 52 ]: proteins are more readily linked to functional differences than transcripts.
Dopamine and Alcohol
Dopamine (DA) is a monoamine transmitter that mediates motivation, attention, short-term memory and reinforcement. Many dopaminergic neurones originate in the ventral tegmental area (VTA) and project to the cerebral cortex, nucleus accumbens (NAc) and amygdala [ 53 ]. The mesolimbic system of the brain has been a focus of addiction research because it involves the so-called pleasure-centers such as the NAc. Dopaminergic transmission in this system may play a central role in many, if not all, addictions [ 54 ]. Animal studies show a dose-dependent increase in DA in response to alcohol in the NAc, indirectly mediated via the VTA. An increase in DA in the NAc occurs in conditioned animals in anticipation of ethanol administration. The local application of dopaminergic-specific neurotoxins in the NAc can lead to a reduction in ethanol consumption in alcohol-dependent rats. In human subjects, PET studies show a significant decrease in DA D 2 receptor binding in alcoholics. Alcoholics also show reduced dopaminergic function that correlates with addiction severity. Despite these observations, DA agonists and antagonists have had limited success as treatments for alcoholism [ 55 ].
Genetic variation in DRD2
The DRD2 gene that encodes the D 2 receptor has several well-characterized polymorphisms, some of which have been associated with diseases such as schizophrenia and dependence. The Taq IA SNP has been extensively studied in relation to alcohol misuse. There is, however, little agreement whether it is [ 56 - 64 ] or is not [ 65 - 69 ] associated with alcohol dependence. This work is further complicated by some studies being limited to special populations [ 66 , 68 ], limited to a single gender [ 61 , 64 ], or broadened to additional psychiatric disorders [ 58 , 60 , 64 ]. Meta-analyses also disagree, with some finding an association with alcohol dependence [ 70 , 71 ] and others not [ 72 ]. The Taq IA story was further complicated when it was found that the SNP, which is ca. 9 kbp downstream of the last exon of DRD2 , is in fact in the coding sequence of a poorly characterized neighboring gene, ankyrin-repeat containing kinase 1 ( ANKK1 ) [ 73 ]. Work in this area continues.
CURRENT TRENDS IN METHAMPHETAMINE USE AND ABUSE
Abuse of methamphetamine (METH) is a growing international public health problem with an estimated 35 million users worldwide, including countries like Canada, China, Japan, Mexico, and USA [ 74 ]. It is thought that over half of the world’s METH consumers reside in Southeast Asia. In Mexico, the number of people admitted to treatment for psychostimulant addiction from 3% in 1996 to 20% in 2006. METH is the most commonly synthesized illegal drug in the United States and has been cited by law enforcement officials as the leading cause of criminal problems in the country. A 2006 survey showed that 5.8% of Americans aged 12 years or older used METH at least once [ 75 ]. There have been substantial increases in METH-related emergency room admissions at hospitals in the Southwest of the USA.
After taking the drug, users experience a sense of euphoria, increased productivity, hypersexuality, decreased anxiety and increased energy. These effects can last for several hours. METH abuse is also associated with a number of negative which include acute toxicity, altered behavioral and cognitive functions, and neurological damage [ 76 ]. Ingestions of large doses of the drug can also cause more serious consequences that include life-threatening hyperthermia, renal and liver failure, cardiac arrhythmias, heart attacks, cerebrovascular hemorrhages, strokes and seizures. Chronic abuse of METH contributes to anxiety, depression, aggressiveness, social isolation, psychosis, mood disturbances, and psychomotor dysfunction. Withdrawal from METH can produce anhedonia, irritability, fatigue, impaired social functioning, and intense craving for the drug [ 76 ]. Neuroimaging studies have revealed METH-induced neurodegenerative changes in the brains of human addicts [ 77 ]. These include persistent decreases in the levels of dopamine transporters (DAT) in various brain. Structural magnetic resonance imaging (MRI) studies in METH addicts have documented substantial morphological changes in their brains [ 78 ].
Studies in animal models have reported that METH can cause depletion of dopamine, serotonin, and of their metabolites in the brain [ 79 ]. These abnormalities are associated with marked decreases in the DA and 5-HT transporters in various brain regions. These abnormalities are thought to be related to the production of oxygen-based radicals including superoxide radicals, hydrogen peroxide, and hydroxyl radicals. Damage to mitochondria and abnormal metabolism of other reactive compounds might also play a role in causing METH-induced damage in monoaminergic terminals [ 79 ]. Some of the damage are prevented by pretreatment with dopaminergic receptor blockers and trophic factors such as GDNF or BMP7 [ 80 ]. Another process that has shown to be protective involves administration of low doses for METH that are not toxic, suggesting that small doses of the drug can trigger molecular and cellular changes that render the brain refractory to its pro-oxidant properties [ 81 ]. If we can project this idea to the human condition, this process might explain why drug addicts do not develop signs and symptoms of Parkinsonism.
In summary, METH addiction is a major neuropsychiatric problem. Research is under way in order to understand the basic mechanisms involved in switching from exposure to drug to being addicted to METH. These studies involve behavioral, cellular and molecular neurobiological approaches. It is hoped that understanding of the pathways involved will lead to better treatment approaches to the clinical population of METH addicted individuals.
TIME TO TAKE A CLOSER LOOK AT MDMA USE/ABUSE
MDMA became a popular drug in the USA in the 1970s and its use rapidly spread to Europe. Initially, drug use was associated with "raves" and the youth dance culture and in the early 1990s reports of excessive use were restricted to case studies [ 82 - 85 ]. Early surveys suggested that use was limited [ 86 , 87 ] and the seeming ability to control intake led to the belief that the drug had limited abuse potential [ 88 , 89 ] did “not seem addictive” [ 90 ] and had “almost no potential to lead to dependence” [ 91 ]. In 1995, the Monitoring the Future survey in the USA indicated an increase in ecstasy use among high school students. subsequent surveys indicated an increase in prevalence rates through to 2001 followed by a decrease. Current prevalence rates (2007) are at about 4% of high school students in the USA. The pattern of ecstasy use also appears to have changed quite considerably in more recent years. While most users consume MDMA relatively infrequently, increasingly the data indicate that many users consume MDMA frequently and in large amounts and some users met criteria for abuse and/or dependence as measured by DSM. The changes being observed in MDMA use and abuse are reminiscent of those that were seen with cocaine in the 1980s. Cocaine was also not initially considered to be “addictive” [ 92 ], a conclusion that we now know is not true. Some cocaine users consume the drug in a compulsive manner that characterizes abuse. MDMA users have also been classified as either novice or light users, moderate users or heavy users based upon either length of time of use, number of pills typically ingested per use or total lifetime use. When one examines the use patterns, it becomes apparent that heavy users take more pills on each occasion [ 93 - 96 ]. Specifically, the number of tablets usually taken and the largest number of tablets ever taken on one occasion was largest in the subjects classified as heavy MDMA users.
There is considerable controversy about the extent of short- and long-term consequences of MDM use and abuse. We have several decades of preclinical research that has investigated self-administration and other models of drug abuse. This knowledge and paradigm development is being applied to the study of MDMA abuse to ultimately understand the neurobiological mechanisms and the consequences of use and abuse of this drug.
ACKNOWLEDGEMENTS
Dr. Onaivi acknowledges financial support from William Paterson University center for research, the Dean, Dr. Sandra DeYoung for continued student worker support and the Provost office for release time. Dr. Dodd’s group in Australia are grateful to Neuropathologists from the Queensland Brain Bank, SCMB, University of Queensland, and from the NSW Tissue Resource Centre, for providing tissue samples; and to the next of kin, for informed written consent for the studies. The tissue banks are part of Australian Brain Bank Network supported by the National Health and Medical Research Council (NHMRC). The NSW Centre and Australian Brain Donor Program are supported by The University of Sydney, NHMRC, Schizophrenia Research Institute, National Institutes of Alcoholism and Alcohol Abuse USA (NIAAA), and NSW Department of Health. Financial support was provided by the NIAAA under grant NIH AA12404 and the NHMRC under grant #401551. Work in Dr. Cadet’s laboratory is supported by the DHHS/NIH/NIDA Intramural Research Program. Dr. Schenk’s studies on the behavioral and neuro-chemical consequences of MDMA self-administration are supported by the Neurological Foundation of New Zealand and the Royal Society of New Zealand Marsden Fund. Dr. Koob was supported by the Pearson Center for Alcoholism and Addiction Research and National Institutes of Health grants AA013517 and AA006420 from the National Institute on Alcohol Abuse and Alcoholism and DA010072, DA004398, and DA023597 from the National Institute on Drug Abuse.
- 1. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Uhl GR, Drgon T, Johnson C, Li C-Y, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann. N.Y. Acad. Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Onaivi ES. Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int. Rev. Neurobiol. 2009;88:335–369. doi: 10.1016/S0074-7742(09)88012-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Onaivi ES, Leonard CM, Ishiguro H, Zhang P-W, Lin Z, Akinshola BE, Uhl GR. Endocannabinoids and cannabinoid receptor genetics. Prog. Neurobiol. 2002;66:307–344. doi: 10.1016/s0301-0082(02)00007-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum. Mol. Genet. 2005;14:3389–3396. doi: 10.1093/hmg/ddi370. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J. Leukoc. Biol. 2005;78:231–238. doi: 10.1189/jlb.0205111. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Lu AT, Ogdie MN, Järvelin MR, Moilanen IK, Loo S-K, McCracken JT, McGough JJ, Yang M-H, Peltonen L, Nelson SF, Cantor RM, Smalley SL. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am. J. Med. Genet. B Neuropsychiat. Genet. 2008;147:1488–1489. doi: 10.1002/ajmg.b.30693. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int. J. Obes. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Jesudason D, Wittert G. Endocannabinoid system in food intake and metabolic regulation. Curr. Opin. Lipidol. 2008;19:344–348. doi: 10.1097/MOL.0b013e328304b62b. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Serra G, Fratta W. A possible role for the endocannabinoid system in the neurobiology of depression. Clin. Pract. Epidemiol. Ment. Health. 2007;3(25):1–11. doi: 10.1186/1745-0179-3-25. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Onaivi ES, Ishiguro H, Gong J-P, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N.Y. Acad. Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Palomo T, Kostrzewa RM, Beninger RJ, Archer T. Genetic variation and spared biological susceptibility underlying comorbidity in neuropsychiatry. Neurotox. Res. 2007;12:29–42. doi: 10.1007/BF03033899. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Mathers C, Vos T, Stevenson C. The burden of injury and disease in Australia. Canberra: AIHW; 1999. [ Google Scholar ]
- 14. WHO. Global Status Report on Alcohol. Canberra: Department of Mental Health and Substance Abuse, WHO; 2004. [ Google Scholar ]
- 15. AIHW. 2001 National Drug Strategy Household Survey: First results. Drug Statistics Series. Vol. 9. Canberra: Australian Institute of Health and Welfare; 2002. [ Google Scholar ]
- 16. Collins DJ, Lapsley HM. Counting the cost: estimates of the social costs of drug abuse in Australia in 1998-9. Canberra: Commonwealth Department of Health and Ageing; 2002. [ Google Scholar ]
- 17. AIHW. Statistics on drug use in Australia 2002. Canberra: Australian Institute of Health and Welfare; 2003. [ Google Scholar ]
- 18. Harper CG, Kril JJ. Neuropathological changes in alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage; NIH Publications: Rock. Rockville, MD: NIH Publications; 1993. pp. 39–69. [ Google Scholar ]
- 19. Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol. (Berlin) 1989;79:200–204. doi: 10.1007/BF00294379. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Saunders JB, Latt N. Epidemiology of alcoholic liver disease. In: Hayes PC, editor. Clinical Gastroenterology. Sydney: Ballière Tindall; 1993. pp. 555–579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Dodd PR, Williams SH, Gundlach AL, Harper PAW, Healy PJ, Dennis JA, Johnston GAR. Glutamate and γ-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J. Neurochem. 1992;59:582–590. doi: 10.1111/j.1471-4159.1992.tb09409.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh E-W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin. Exp. Res. 2005;29(8):1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin. Exp. Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–134. [ PubMed ] [ Google Scholar ]
- 27. Snell LD, Tabakoff B, Hoffman PL. Radioligand binding to the N-methyl-D-aspartate receptor/ionophore complex: alterations by ethanol in vitro and by chronic in vivo ethanol ingestion. Brain Res. 1993;602:91–98. doi: 10.1016/0006-8993(93)90246-j. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Haugbol SR, Ebert B, Ulrichsen J. Upregulation of glutamate receptor subtypes during alcohol withdrawal in rats. Alcohol Alcohol. 2005;40(2):89–95. doi: 10.1093/alcalc/agh117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Sircar R, Sircar D. Repeated ethanol treatment in adolescent rats alters cortical NMDA receptor. Alcohol. 2006;39(1):51–58. doi: 10.1016/j.alcohol.2006.07.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Devaud LL, Morrow AL. Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus. Eur. J. Pharmacol. 1999;369(3):331–334. doi: 10.1016/s0014-2999(99)00103-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Darstein MB, Landwehrmeyer GB, Feuerstein TJ. Changes in NMDA receptor subunit gene expression in the rat brain following withdrawal from forced long-term ethanol intake. Naunyn-Schmiedeberg s Arch. Pharmacol. 2000;361(2):206–213. doi: 10.1007/s002109900180. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin. Exp. Res. 2004;28(6):957–965. doi: 10.1097/01.alc.0000128225.83916.40. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Nixon K, Hughes PD, Amsel A, Leslie SW. NMDA receptor subunit expression after combined prenatal and postnatal exposure to ethanol. Alcohol Clin. Exp. Res. 2004;28(1):105–112. doi: 10.1097/01.ALC.0000106311.88523.7B. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Res. 2005;1048(1-2):69–79. doi: 10.1016/j.brainres.2005.04.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Michaelis EK, Freed WJ, Galton N, Foye J, Michaelis ML, Phillips I, Kleinman JE. Glutamate receptor changes in brain synaptic membranes from human alcoholics. Neurochem. Res. 1990;15:1055–1063. doi: 10.1007/BF01101704. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Freund G, Anderson KJ. Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin. Exp. Res. 1996;20(7):1165–1172. doi: 10.1111/j.1530-0277.1996.tb01106.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Ridge JP, Ho AM.-C, Innes DJ, Dodd PR. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann. NY Acad. Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Ridge JP, Dodd PR. Glutamate and ethanol: the role of ionotropic NMDA glutamate receptors in alcoholism. In: Paley BF, Warfield TE, editors. In: Amino Acid Receptor Research. New York: Nova Science Publishers; 2008. pp. 367–377. [ Google Scholar ]
- 39. Ridge JP, Dodd PR. Cortical NMDA receptor expression in human chronic alcoholism: influence of the TaqIA allele of ANKK1. Neurochem. Res. 2009;34:1775–1782. doi: 10.1007/s11064-009-9941-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Ridge JP, Ho AM-C, Dodd PR. Sex differences in NMDA receptor expression in human alcoholics. Alcohol Alcohol. 2009;44:594–601. doi: 10.1093/alcalc/agp052. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Arstad E, Platzer S, Berthele A, Pilowsky LS, Luthra SK, Wester HJ, Henriksen G. Towards NR2B receptor selective imaging agents for PET — synthesis and evaluation of N-[11C]-(2-methoxy)benzyl (E)-styrene-, 2-naphthyl- and 4-trifluoro-methoxyphenylamidine. Bioorg. Med. Chem. 2006;14(18):6307–6313. doi: 10.1016/j.bmc.2006.05.046. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Stone JM, Erlandsson K, Ärstad E, Bressan RA, Squassante L, Teneggi V, Ell PJ, Pilowsky LS. Ketamine displaces the novel NMDA receptor SPET probe [123I]CNS- 1261 in humans in vivo. Nucl. Med. Biol. 2006;33(2):239–243. doi: 10.1016/j.nucmedbio.2005.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Matsumoto R, Haradahira T, Ito H, Fujimura Y, Seki C, Ikoma Y, Maeda J, Arakawa R, Takano A, Takahashi T, Higuchi M, Suzuki K, Fukui K, Suhara T. Measurement of glycine binding site of N-methyl-D-asparate receptors in living human brain using 4-acetoxy derivative of L-703, 717, 4-Acetoxy-7-chloro-3-[3-(4-[11C] methoxybenzyl) phenyl]-2(1H)-quinolone (AcL703) with positron emission tomography. Synapse. 2007;61(10 ):795–800. doi: 10.1002/syn.20415. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Liu J, Lewohl JM, Dodd PR, Randall PK, Harris RA, Mayfield RD. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J. Neurochem. 2004;90:1050–1058. doi: 10.1111/j.1471-4159.2004.02570.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31(7 ):1574–1582. doi: 10.1038/sj.npp.1300947. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Etheridge N, Lewohl JM, Mayfield RD, Harris RA, Dodd PR. Synaptic proteome changes in the superior frontal gyrus and occipital cortex of the alcoholic brain. Proteomics Clin. Appl. 2009;3:730–742. doi: 10.1002/prca.200800202. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AEG, Harper CG, Dodd PR. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Ann. NY Acad. Sci. 2004;1025:39–46. doi: 10.1196/annals.1316.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Bergeson SE, Berman AE, Dodd PR, Edenberg HJ, Hitzemann RJ, Lewohl JM, Lodowski KH, Sommer W. Expression profiling in alcoholism research. Alcohol. Clin. Exp. Res. 2005;29:1066–1073. doi: 10.1097/01.ALC.0000171043.29384.3E. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Dodd PR, Buckley ST, Eckert AL, Foley PF, Innes DJ. Genes and gene expression in the brains of human alcoholics. Ann. N.Y. Acad. Sci. 2006;1074:104–115. doi: 10.1196/annals.1369.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Ponomarev I, Schafer GL, Blednov YA, Williams RW, Iyer VR, Harris RA. Convergent analysis of cDNA and short oligomer microarrays, mouse null mutants and bioinformatics resources to study complex traits. Genes Brain Behav. 2004;3:360–368. doi: 10.1111/j.1601-183x.2004.00088.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health. 2007;30(1 ):5–13. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Di Chiara G. Alcohol and dopamine. Alcohol Health Res. World. 1997;21(2 ):108–114. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006;30(2 ):215–238. doi: 10.1016/j.neubiorev.2005.04.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28(8):1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Noble EP, Syndulko K, Fitch RJ, Ritchie T, Bohlman MC, Guth P, Sheridan PJ, Montgomery A, Heinzmann C, Sparkes RS, Kenneth B. D2 dopamine receptor TaqI A alleles in medically ill alcoholic and nonalcoholic patients. Alcohol Alcohol. 1994;29(6 ):729–744. [ PubMed ] [ Google Scholar ]
- 57. Ishiguro H, Arinami T, Saito T, Akazawa S, Enomoto M, Mitushio H, Fujishiro H, Tada K, Akimoto Y, Mifune H, Shioduka S, Hamaguchi H, Toru M, Shibuya H. Association study between the -141C Ins/Del and TaqI A polymorphisms of the dopamine D2 receptor gene and alcoholism. Alcohol. Clin. Exp. Res. 1998;22(4 ):845–848. [ PubMed ] [ Google Scholar ]
- 58. Lu RB, Lee JF, Ko HC, Lin WW. Dopamine D2 receptor gene (DRD2) is associated with alcoholism with conduct disorder. Alcohol Clin. Exp. Res. 2001;25(2 ):177–184. [ PubMed ] [ Google Scholar ]
- 59. Madrid GA, MacMurray J, Lee JW, Anderson BA, Comings DE. Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism. Alcohol. 2001;23(2):117–122. doi: 10.1016/s0741-8329(00)00138-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Young RM, Lawford BR, Noble EP, Kann B, Wilkie A, Ritchie T, Arnold L, Shadforth S. Harmful drinking in military veterans with post-traumatic stress disorder: association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002;37(5 ):451–456. doi: 10.1093/alcalc/37.5.451. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Hallikainen T, Hietala J, Kauhanen J, Pohjalainen T, Syvalahti E, Salonen JT, Tiihonen J. Ethanol consumption and DRD2 gene TaqI a polymorphism among socially drinking males. Am. J. Med. Genet. A. 2003;119A(2 ):152–155. doi: 10.1002/ajmg.a.20139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Berggren U, Fahlke C, Aronsson E, Karanti A, Eriksson M, Blennow K, Thelle D, Zetterberg H, Balldin J. The taqI DRD2 A1 allele is associated with alcohol-dependence although its effect size is small. Alcohol Alcohol. 2006;41(5 ):479–485. doi: 10.1093/alcalc/agl043. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, Budde JP, Goldstein EL, Kaplan D, Edenberg HJ, Nurnberger J, Jr, Hesselbrock V, Schuckit M, Kuperman S, Tischfield J, Porjesz B, Begleiter H, Bierut LJ, Goate A. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcohol Clin. Exp. Res. 2007;31(10):1645–1653. doi: 10.1111/j.1530-0277.2007.00470.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Ponce G, Hoenicka J, Jimenez-Arriero MA, Rodriguez-Jimenez R, Aragues M, Martin-Sune N, Huertas E, Palomo T. DRD2 and ANKK1 genotype in alcohol-dependent patients with psychopathic traits: association and interaction study. Br. J. Psychiatry. 2008;193(2):121–125. doi: 10.1192/bjp.bp.107.041582. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Gelernter J, O’Malley S, Risch N, Kranzler HR, Krystal J, Merikangas K, Kennedy JL, Kidd KK. No association between an allele at the D2 dopamine receptor gene (DRD2) and alcoholism. JAMA. 1991;266(13):1801–1807. [ PubMed ] [ Google Scholar ]
- 66. Chen CH, Chien SH, Hwu HG. Lack of association between TaqI A1 allele of dopamine D2 receptor gene and alcohol-use disorders in atayal natives of Taiwan. Am. J. Med. Genet. C Sem. Med. Genet. 1996;67C(5):488–490. doi: 10.1002/(SICI)1096-8628(19960920)67:5<488::AID-AJMG10>3.0.CO;2-J. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Matsushita S, Muramatsu T, Murayama M, Nakane J, Higuchi S. Alcoholism, ALDH2*2 allele and the A1 allele of the dopamine D2 receptor gene: an association study. Psychiatry Res. 2001;104(1 ):19–26. doi: 10.1016/s0165-1781(01)00290-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Shaikh KJ, Naveen D, Sherrin T, Murthy A, Thennarasu K, Anand A, Benegal V, Jain S. Polymorphisms at the DRD2 locus in early onset alcohol dependence in the Indian population. Addict. Biol. 2001;6:331–335. doi: 10.1080/13556210020077055. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum. Mol. Genet. 2007;16(23):2844–2853. doi: 10.1093/hmg/ddm240. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am. J. Med. Genet. B Neuropsychiat. Genet. 2003;116B(1):103–125. doi: 10.1002/ajmg.b.10005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Young RM, Lawford BR, Nutting A, Noble EP. Advances in molecular genetics and the prevention and treatment of substance misuse: implications of association studies of the A1 allele of the D2 dopamine receptor gene. Addict. Behav. 2004;29:1275–1294. doi: 10.1016/j.addbeh.2004.06.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Munafò MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol. Psychiatry. 2007;12(5):454–461. doi: 10.1038/sj.mp.4001938. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum. Mutat. 2004;23(6 ):540–545. doi: 10.1002/humu.20039. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. UNODC. World Drug Report Volume 1. Analysis. Volume 1. Vienna: United Nations Office on Drugs and Crime; 2007. [ Google Scholar ]
- 75. SAMHSA. The NSDUH Report: Methamphetamine Use. Rockville, MD: Office of Applied Studies; 2007. [ Google Scholar ]
- 76. Gold MS, Kobeissy FH, Wang KK, Merlo LJ, Bruijnzeel AW, Krasnova IN, Cadet JL. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol. Psychiatry. 2009;66:118–127. doi: 10.1016/j.biopsych.2009.02.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:79–407. doi: 10.1016/j.brainresrev.2009.03.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Chou J, Luo Y, Kuo C-C, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience. 2008;151:92–103. doi: 10.1016/j.neuroscience.2007.10.044. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Cadet JL, McCoy MT, Cai N-S, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One. 2009;4(11 ):e7812. doi: 10.1371/journal.pone.0007812. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. McGuire P, Fahy T. Chronic paranoid psychosis after misuse of MDMA (“ecstasy”) Br. Med. J. 1991;302:697. doi: 10.1136/bmj.302.6778.697. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Winstock AR. Chronic paranoid psychosis after misuse of MDMA. Br. Med. J. 1991;302:1150–1151. doi: 10.1136/bmj.302.6785.1150-b. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Shearman JD, Chapman RW, Satsangi J, Ryley NG, Weatherhead S. Misuse of ecstasy. Br. Med. J. 1992;305:309. doi: 10.1136/bmj.305.6848.309. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Gorard DA, Davies SE, Clark ML. Misuse of ecstasy. Br. Med. J. 1992;305:309–0. doi: 10.1136/bmj.305.6848.309-a. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Peroutka SJ, Newman H, Harris H. subjective effects of 3, 4-methylenedioxymethamphetamine in recreational users. Neuropsychopharmacology. 1988;1:273–277. [ PubMed ] [ Google Scholar ]
- 87. Cohen RS. subjective reports on the effects of the MDMA (‘ecstasy’) experience in humans. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19:1137–1145. doi: 10.1016/0278-5846(95)00231-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Solowij N, Hall W, Lee N. Recreational MDMA use in Sydney: a profile of 'Ecstacy' users and their experiences with the drug. Br. J. Addict. 1992;87:1161–1172. doi: 10.1111/j.1360-0443.1992.tb02003.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Beck J, Rosenbaum M. Pursuit of Ecstasy: The MDMA Experience. Albany: State University of New York Press; 1994. [ Google Scholar ]
- 90. Henry JA. Ecstasy and the dance of death. Br. Med. J. 1992;305:5–6. doi: 10.1136/bmj.305.6844.5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Hammersley R, Ditton J, Smith I, Short E. Patterns of ecstasy use by drug users. Br. J. Criminol. 1999;39:625–647. [ Google Scholar ]
- 92. Washington AM, Gold MS. Recent rends in cocaine abuse: A view from the national hotline 800-COCAINE. In: Stimmel B, editor. In: Cocaine: Pharmacology Addiction and Therapy. New York, NY: Haworth Press; pp. 31–48. [ Google Scholar ]
- 93. Scholey AB, Parrott AC, Buchanan T, Heffernan TM, Ling J, Rodgers J. Increased intensity of Ecstasy and polydrug usage in the more experienced recreational Ecstasy/MDMA users: a WWW study. Addict. Behav. 2004;29:743–752. doi: 10.1016/j.addbeh.2004.02.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Verkes RJ, Gijsman HJ, Pieters MS, Schoemaker RC, de Visser S, Kuijpers M, Pennings EJ, de Bruin D, van de Wijngaart G, van Gerven JM, Cohen AF. Cognitive performance and serotonergic function in users of ecstasy. Psychopharmacology. 2001;153:196–202. doi: 10.1007/s002130000563. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Fox HC, Parrott AC, Turner JJ. Ecstasy use: cognitive deficits related to dosage rather than self-reported problematic use of the drug. J. Psychopharmacol. 2001;15:273–281. doi: 10.1177/026988110101500406. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Parrott AC. Chronic tolerance to recreational MDMA (3, 4-methylenedioxymethamphetamine) or Ecstasy. J. Psychopharmacol. 2005;9:71–83. doi: 10.1177/0269881105048900. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (81.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
Feb 10, 2018 · PDF | Drug abuse, also known as Drug addiction is defined as chronic, relapsing brain disease that is characterized by compulsive drug seeking and use,... | Find, read and cite all the research ...
Nov 23, 2017 · The current paper highlights the causes of drug abuse, and describes the treatment and prevention of drug abuse and addiction for proper management of the problem. Discover the world's research 25 ...
According to the 2016 United States Surgeon General’s Report , more than 60% of those who received addiction treatments in the United States relapsed within a year, which highlights the challenges in sustaining recovery (i.e., maintaining long-term drug abstinence and well-being). Despite decades of scientific research and the high economic ...
Nov 14, 2023 · A drug is any substance capable of altering the functioning of a person’s body and mind. In this paper, a deterministic nonlinear model was adapted to investigate the behavior of drug abuse and ...
Nov 1, 2020 · Addiction is the key process that underlies substance use disorders, and research using animal models and humans has revealed important insights into the neural circuits and molecules that mediate addiction. More specifically, research has shed light onto mechanisms underlying the critical components of addiction and relapse: reinforcement and ...
Abstract. This paper first introduces important conceptual and practical distinctions among three key terms: substance “use,” “misuse,” and “disorders” (including addiction), and goes on to describe and quantify the important health and social problems associated with these terms.
Oct 19, 2020 · A schematic neurocognitive model illustrating a proposed functional role for three key neural systems in addiction: The first two systems include the competing-neurobehavioral-decision-systems (CNDS) model, which consists of an impulsive system that includes the amygdala and its connections to the ventral and dorsal striatum, and their associated mesolimbic dopamine systems.
Aug 12, 2021 · The characterisation of the role of glia and the extracellular matrix (ECM) in drug-induced synaptic plasticity is an exciting emerging field of drug addiction research as it comes with promising ...
These research queries and findings are presented in the form of updates, white papers and case studies. In addition, the Butler Center for Research collaborates with the Recovery Advocacy team to study special-focus addiction research topics, summarized in monthly Emerging Drug Trends reports. Altogether, these studies provide the latest in ...
Keywords: International Drug Abuse Research Society, IDARS, addiction, alcohol, marijuana, psychostimulants. INTRODUCTION. Drug addiction is a chronically relapsing disorder that has been characterized by the compulsive use of addictive substances despite adverse consequences to the individual and society . Addiction to drugs and alcohol is ...