Determination of Planck’s constant.
0-10 V power supply, a one way key, a rheostat, a digital milliammeter, a digital voltmeter, a 1 K resistor and different known wavelength LED’s (Light-Emitting Diodes).
Planck’s constant (h), a physical constant was introduced by German physicist named Max Planck in 1900. The significance of Planck’s constant is that ‘quanta’ (small packets of energy) can be determined by frequency of radiation and Planck’s constant. It describes the behavior of particle and waves at atomic level as well as the particle nature of light.
An LED is a two terminal semiconductor light source. In the unbiased condition a potential barrier is developed across the p-n junction of the LED. When we connect the LED to an external voltage in the forward biased direction, the height of potential barrier across the p-n junction is reduced. At a particular voltage the height of potential barrier becomes very low and the LED starts glowing, i.e., in the forward biased condition electrons crossing the junction are excited, and when they return to their normal state, energy is emitted. This particular voltage is called the knee voltage or the threshold voltage . Once the knee voltage is reached, the current may increase but the voltage does not change.
The light energy emitted during forward biasing is given as ,
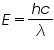
c -velocity of light. h -Planck’s constant. λ -wavelength of light.
If V is the forward voltage applied across the LED when it begins to emit light (the knee voltage), the energy given to electrons crossing the junction is,
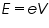
Equating (1) and (2), we get
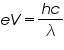
The knee voltage V can be measured for LED’s with different values of λ (wavelength of light).
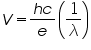
To determine Planck’s constant h, we take the slope s from our graph and calculate
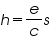
using the known value
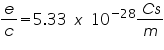
Alternatively, we can write equation (3) as
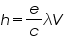
calculate h for each LED, and take the average of our results.
Cite this Simulator:
Apni Physics

Tuesday, January 22nd, 2019
Dr Sushil Kumar

Planck’s Constant Experiment Excellent 15 Viva Questions
experiment , Physics , planck constant experiment viva , planck constant experiment viva voce , planck constant viva questions , planck's constant experiment viva questions , planck's constant experiment viva questions with answers , planck's constant experiment viva questions with answers pdf , planck's constant viva questions , Viva , viva questions for planck's constant experiment
Last updated on Sunday, October 15th, 2023
Planck’s Constant Experiment Viva Questions:
Table of Contents
Planck’s Constant using LED
Determination of planck’s constant by using light-emitting diodes (leds)..
APPARATUS: Planck’s constant kit, wires, graph paper, and 3-4 LEDs.
FORMULA USED: Planck’s constant is h = eV /ν, where e is an electronic charge, V is the voltage reading in a voltmeter, and v is the frequency of a particular LED color.
THEORY: The energy of a photon is given by the equation: E = h ν (1)
Where E is the energy of a photon, ν is its frequency, and ( h) is Planck’s constant.
CASE 1: In the case of the photoelectric effect:
- An electron is emitted from the metal only if the energy of the incident photon is greater than the work function of the metal.
- These electrons can be attracted by the anode in a circuit and as a result of a current, you can observe with respect to the incident radiations (color).
- If you need to measure the voltage difference, you can use resistance (the choice is open) in the circuit.
- This voltage will be corresponding to the particular incident radiation energy.
- If you need to know any other parameters, you can use this information (the current, the voltage, the color of incident radiation means the wavelength or frequency, so you can find the E=hv of the incident photon).
READ ALSO: Semiconductor Diode Laser Viva
Case 2. in the case of leds, the opposite of the above-mentioned working is true..
- Here, in LED, If an electron of sufficient electrical energy (eV) is passed across a material then a photon emits.
- But remember, the meaning of passing the electron across a material here is a diode.
- That has two types of semiconductors (n- and p-type) and a p-n junction. Near the p-n junction, there is a specific region known as the depletion region.
- These electrons start from the n-region and after crossing the barrier (depletion region) reach the p-region where they recombine and as a result, a photon emits.
- But to understand it you have to understand the energy band concept, this explanation is given in the semiconductor laser topic.
Planck’s Constant Experiment Supporting Concepts to LED
Please note that all materials don’t show the photoelectric effect and emission of radiation like in LED. For LED we use Ga As the material that shows the optical properties when electron-hole recombination takes place. If you got the flavor of the second case then it will be clear that you need forward biasing for this purpose. When you provide a sufficient voltage to the electron to cross the barrier only then it recombines with holes. And only then you can see the photons means that light. So initially you can not see the light when you provide potential to the LED. But when you reach the threshold value of voltage where the electron is able to cross the junction then only you see the light. This value of potential you know is known as stopping potential. Now the point is clear, also the emitted photon energy (hv) will be the same as the electrical energy of the electron (eV). Because of this reason you use; eV = h ν h = eV / ν This equation we will use to determine Planck’s constant.
PLANCK’S EXPT. PROCEDURE:
- Make the connection in the kit.
- Take the current measurement of each LED by varying the voltage as given in the table.
- Plot the curve on the graph paper between the frequency of color on the X-axis and electrical energy on Y-axis for all LEDs.
- The slope of the curve will give a measured value of Planck’s constant.
- Compare the measured value with the standard value and check the percentage error.
OBSERVATION S:
As mentioned in the procedure plot a graph between the last two-column of the above table that is the frequency of the particular LED and energy (eV). Take the slope of this graph and this will be your measured value of Planck’s constant. Now compare it with the standard value (6.62607004 × 10 -34 m 2 kg / s) and explain the percentage error. Check yourself that what can be the reasons for this percentage error.
What you can analyze in Planck’s Constant Experiment?
From your observation, you can also analyze the stopping potential of all the LEDs. How? Just see you have taken readings for each LED, either that one when the LED starts to glow or also after it with some intervals of potential. So you have a set of reading with potential and current for each LED. When you will plot it for every LED you will observe that every LED starts with a specific value of the potential. This value of the potential is known as the stopping potential and this way, you can analyze the stopping potential for each color LED. But to determine Planck’s constant you will need a graph that points explained above.

How to Observe Planck’s Constant from this Experiment?
- Take current and voltage readings for each LED (Red, Green, and Blue)
- Plot a graph on the graph paper using proper scaling of the variables (Voltage and Current), as shown in the above figure.
- Now find the knee voltage for each one, and make one more graph between stopping potential and frequency as shown in 2nd part of the above picture.
- Take the slope from here and use it in the formula as you can see right side of the above figure.
- Calculate the percentage error by using the formula and check the reasons for it, if more than 20%.
7 -Ways Can Improve Your Learning Habits https://apniphysics.com/students-life/7-ways-improve-life-students/
Strategies that can help students stay motivated and possible ways of distractions https://apniphysics.com/students-life/strategies-students-stay-motivated/
Points of Care when You make the Graphs:
- When you are plotting the graph between any two physical variables of the experiment, first make a scale along the X-axis and Y-axis. For example, 10 small boxes are equal to 1 volt along the Y-axis or 1 mA along the X-axis.
- Then mark the points where it lies
- After it joins all the points. Now at this point keep remembering, it is not necessary that all your points will lie in a straight line. You have to draw a line that passes over to or near each point. Like, I have drawn the line above, which is passing near to that point which was away from it.
- Now you can take any two points on the line except for them which were marked earlier. Find the slope and put it in the required formula. This will be your observed value.
- I hope it will be enough to get my point, for any doubt you can ask through the comment box.
VIVA QUESTIONS Planck’s Constant Experiment
- How Planck’s constant is determined? “Planck’s constant is determined by using the light-emitting diode. The LED works on the principle of semiconductor diode, to flow the current from n to p-type semiconductor a minimum potential is required. When electron reaches the p-type semiconductor they fuse there and emits a photon. So in this way, we can compare the potential energy eV 0 with the energy of the photons h and nu.
Q1. How it is different from Si/Ge diode?
Ans . LED is made of Ga As Gallium Arsenide semiconductor material which shows the optical properties when electron-hole recombination takes place. While Si/Ge-made diode or semiconductor shows thermal properties, they start to heat up when current flows. On the other hand LED glow.
2Q. How LED works?
2Answer: When forward bias gives to the light emitting diode (LED), immediately LED doesn’t glow and takes some time. The minimum potential at which LED starts to glow is known as the stopping potential. The light from LED is the result of electron and hole recombination in the depletion region.
3Q. Why Minimum potential is required to glow the LED?
3Ans. There is a potential barrier for the charge carrier to cross the junction, to overcome it they required this amount of potential energy. And then after with small change in potential, they cross the junction, and current flows through the LED.
4Q. In the photoelectric effect, a suitable frequency of photon falls on an electron in an atom and ejects the electron. In LED when electron-hole recombination takes place a photon emits. How do you see these two phenomena?
4Ans: Both phenomena are different, in the case of the “photoelectric effect” to emit the electron, from the surface of a material, a minimum energy of threshold frequency is required. While on the other hand, in a light-emitting diode (LED) photon emits when electron-hole recombination takes place above the threshold value of the voltage; known as stopping potential.
5Q. Why do you put two different energies eV and hv equal, what is the condition that they satisfy in the LED?
Answer: From the question, no. 3 you understood the stopping potential, and a small potential above it shows the deflection in current, simultaneously glowing in the LED. The potential energy eV is responsible to recombine the electron-hole recombination, by which a photon of the energy hv emits. Because of this reason, one can put eV = hv
6Q.Which material do we use in the LED?
Answer: Gallium Arsenide which is of a semiconductor nature.
7Q. How are photons emitted from the LED and from which section of the LED?
Answer: When electron-hole recombine photons emit, these emit from the depletion region.
8Q. How do you explain the working of LED by using the energy band diagram in forward biasing?
Answer: You have to follow a detailed lecture for this purpose. Click Here Working of LED.
7Q. What happens when you provide the forward bias to the LED in terms of the conduction band & valence band in the depletion region?
Answer: Conduction band and valence band drift in the depletion region, for more info watch this video .
8Q. Why does not LED starts to glow immediately when you provide the forwarding bias to that?
Answer: Because of the potential barrier at the junction for the charge carriers.
9Q. Explain the concept of stopping potential in semiconductor diode by V-I Characteristics.
Answer: In the above figure, it is pointed out for the red, green, and blue LEDs.
10Q. Why does Blue color LED stopping potential greater than the Red color LED?
Answer: from the relation eV 0 =hν
further ν = c/λ so eV 0 =hν
eV 0 =hc/λ in this relation e, h, and c are constants, so you can see V 0 ∝1/λ
for small wavelength, stopping potential is higher than the larger wavelength. As you can see in Table. 1 the blue color wavelength is 475 nm while for the red it is 650 nm.
11Q. Can we achieve the population inversion process in LED too? if yes what is the condition for that? if not then why?
Answer: Highly doped semiconductor material is required, the first condition, so not possible in LED. and the second reason is a threshold current beyond that we achieve population inversion in the semiconductor LASER.
12 Q. What symbol do we use for the Light Emitting Diode?
Answer: Similar to the normal diode but with arrows, which indicate the emission of light
—-|>-
13Q. What information do we get from Planck’s Constant, and how one can say that radiation is in a discrete form of energy?
Answer: Energy is in the discrete form that signifies by the photon energy; hv
Sources/Information Required to Explain/Understand the Experiment :
1. Working of a p-n Junction diode 2. Depletion region Concept/Idea along with potential barrier for Si/Ge 3. Semiconductor Material name that shows the optical property 4. Mainly energy band diagram of the p-n junction diode and how electron transit from n to p side in depletion region in case of forward biasing. 5. The basic idea of electrical and radiation energy so can understand why they keep equal two different energy.
Highlighted points
determination of Planck’s constant using led experiment viva questions discussed
planck’s constant experiment viva questions with answers available for all, planck’s constant using led experiment manual you can create. photocell experiment viva questions with answers ,led viva questions with answers. planck’s constant experiment detailed information by LEDs, led viva questions included as I can observe. determination of Planck’s constant using led pdf. led characteristics experiment, determination of Planck’s constant using photocell experiment pdf. determination of Planck’s constant. led MCQ questions. photoelectric effect viva questions and answers pdf, Boltzmann constant viva questions. planck’s constant experiment manual, planck’s constant experiment pdf. planck’s constant experiment procedure, determination of Planck’s constant experiment. Plancks constant ev Plancks constant experiment, Planck constant experiment, viva led, v-i characteristics of led experiment.
Plank’s constant page in pdf format, Click here: apniphysics_com_viva_viva_questions_plancks_constant
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to email a link to a friend (Opens in new window)
Latest articles
From quantity to quality: redefining institutional approaches to research publication, celebrating teacher’s day: 11 heartfelt quotes to honor their impact, the ultimate guide to choosing the best voice amplifier for teachers.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

Discover more from Apni Physics
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Featured articles

Saturday, September 7th, 2024

Thursday, September 5th, 2024

Wednesday, June 12th, 2024

Educating Physics
2. Determining the Planck Constant
Objectives:
- To be able to determine the Planck constant using different coloured LEDs.
The Planck constant was introduced by Max Planck back in 1900. The significance of Planck’s constant is that a photons energy (a ‘quanta’ of energy) can be determined by frequency of radiation and Planck’s constant.
The Planck constant can be determined with the use of an LED and power source (as well as an Ammeter and Voltmeter for measurements to be taken). The theory is that with the potential difference up high enough to emit radiation from the LED, the energy the electrons are giving the photons is enough to emit them from the device. Due to the nature of how LED’s work, the potential difference across the LED needs to only just be enough to emit photons. If the e.m.f. supplied to the circuit increases beyond this, the voltage goes on to increasing the current around the circuit and so increases the number of electrons, rather than giving energy to the electrons and therefore photons.

The minimum voltage required to emit a photon from an LED is known as the threshold voltage, this value is partly dependent on the colour of light that the LED is emitting.
A circuit required to determine Planck’s constant could look like one of the following;

So providing the frequency is known, Planck’s constant can be determine. The frequency can be determined using a number of methods; sometimes the supplier will give the frequency of the light, otherwise a data sensor could be used or the methods used in diffraction could be utilised.
Doing this practical with a red LED of wavelength 485 nm gave a p.d. of 1.80 V, substituting these values into an appropriate equation gives;
For one value of voltage and wavelength this is not a bad first result – just 0.81 % off the true value.
As this equation has no y-intercept value, we should expect the line of best fit to pass through the origin.
The following graph can be drawn from this data;

Based on the equation shown earlier, the line should pass through the origin (which it almost does);
In order to increase accuracy the experiment should be carried out in a darkened room OR the LED should be viewed through a narrow tube so that the observer can identify more precisely when the LED first begins emitting light (with an increasing voltage), this way a more accurate threshold voltage can be determined.
CAUTION – In doing this, the voltage can be turned up to quickly and the LED may illuminate very brightly and directly into the users eye.
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
Hello there, haven’t we seen you before
New here? Sign Up
Already have an account? Sign In
You must be logged in to post a comment.
Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric...
Andrea Checchetti, Alessandro Fantini

Science and Education Publishing
From Scientific Research to Knowledge
- Browse by Subjects
- Journal Home
- For Authors
- Online Submission
- Current Issue

Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric Effect

1 Istituto d'Istruzione Superiore "Leonardo da Vinci"
2 Liceo Scientifico "E. Fermi" Catanzaro Lido
- Related Content
- About the Authors
- Follow the Authors
The aim of this experiment was to utilize the Inquiry Based Science Education, (IBSE) in order to offer the students a better understanding of the light-matter interaction. The inquiry-based teaching addresses the question of how the students make observations, develop hypotheses about phenomena, and devise tests to investigate their hypotheses. Using IBSE they share responsibility within the group for what concerns answering questions, and use a scientific approach to solve problems. 4 th year students of undergraduate degree program of an Italian Arts Lyceum and 5 th year students of undergraduate degree program of an Italian Scientific Lyceum have experimentally determined the value of Planck's constant, h . They have used respectively two methods: a) current-voltage measurements of a series of different colored LEDs; b) a method based upon the comparison of the incident light beams values of potential arrest with different wavelength on a photo emissive surface, using the photoelectric effect through an apparatus built on an optical bench. This work is an essential part of an apprenticeship developed during the “Professione Formatore in Didattica delle Scienze” Master, which takes place at the University of Calabria, with the authors taking part in it as trainees and that will be over in the month of October 2015.
Keywords: IBSE (Inquiry-Based Science Education), planck’s constant, LEDs (Light-emitting diodes), photoelectric effect
Received July 10, 2015; Revised August 03, 2015; Accepted August 06, 2015
Cite this article:
- Normal Style
- Chicago Style
- Andrea Checchetti, Alessandro Fantini. Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric Effect. World Journal of Chemical Education . Vol. 3, No. 4, 2015, pp 87-92. https://pubs.sciepub.com/wjce/3/4/2
- Checchetti, Andrea, and Alessandro Fantini. "Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric Effect." World Journal of Chemical Education 3.4 (2015): 87-92.
- Checchetti, A. , & Fantini, A. (2015). Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric Effect. World Journal of Chemical Education , 3 (4), 87-92.
- Checchetti, Andrea, and Alessandro Fantini. "Experimental Determination of Planck’s constant using Light Emitting Diodes (LEDs) and Photoelectric Effect." World Journal of Chemical Education 3, no. 4 (2015): 87-92.
At a glance: Figures

View all figures
1. Introduction
The interaction of light and matter is synthetically expressed by Einstein-Planck equation:
where the constant of proportionality h has the following value:
It represents a milestone in the introduction to quantum mechanics; also known as “quantum of energy” or “quantum of action”. It is one of the fundamental physics constants, useful to understand the meaning of quantization in the atomic and nuclear world and represents the fundamental unit of action for discrete atomic scale system; it defines the minimum amount for the light energy and the electrons’ energies in atoms. Understand its physical meaning is the first step to bring students closer to the concept of quantization of energy, the starting point of a historical-didactic route that has as successive steps the wave nature of the electron, the uncertainty principle, the Schrödinger equation, until trace the development of the atomic structure. Light, quanta and Planck's constant are concepts that embrace both the chemistry that the physics.
The value of h was performed using Light Emitting Diodes (LEDs) and photoelectric effect.
In the Light Emitting Diodes (LEDs) method’s, we first make a complete circuit, using various apparatus (Figure 1). We use four colors (red, yellow, green and blue) for the different LEDs in the first place, to derive the voltage-current characteristic curves using the same circuit (Figure 3).
Then we use the linear part of voltage-current characteristic curves to estimate the slope (m) and intercept (n) corresponding to each LEDs (e.g., Figure 7 shows the red LED). These values are used to estimate V g for different color LEDs, the potential that corresponds to the voltage for which the electrons are able to recombine with “holes” of the valence band. The Planck’s constant corresponding to each color is then determined using equation (3), whose values are presented in Table 2 .
In the Photoelectric Effect method’s, light of a known frequency is allowed to fall on a metal photoemission surface acting as the photocathode (emitter plate in Fig. 6). Electrons are ejected from the cathode and some reach the collector producing a photoelectric current (Fig. 6).
The anode can be made negative (reverse bias) with respect to the cathode to repel the electrons. Only electrons leaving the emitter surface with initial kinetic energy greater than the potential energy between the cathode and collector will reach the anode and register a current. The potential difference can be increased until no electrons reach the collector and the current is stopped. This potential is called the stopping potential V a (eq. 6).
The above operation is repeated for two different radiations (incident light with differing frequencies) so that h can be estimated using equation 7.
The Planck’s constant value has been determined by using, under the educational profile, the same methodology: the Inquiry Based Science Education, (IBSE).
IBSE is a useful process that allows:
• Creation or choice of activities that motivate and involve students
• Determination of learning outcomes and appropriate assessment methods
• Use of specialized tools and digital technology
• Selection of educational resources, such as reading materials and useful websites
The teaching based on the research addresses the matter of how students make observations, how they develop hypotheses about the phenomena and how they can anticipate tests to investigate their hypothesis.
The inquiry process involved the following steps [ 1 ] :
1) Engagement
4) Elaborate
5) Evaluate
For each of the experimental methods used by the authors to calculate the Planck constant, the following Learning Activities have been developed:
LIGHT EMITTING DIODES (LEDS) METHOD
1 st Learning Activity (Developing the concept so that the students can gain an appreciation of the following)
The diode LED, as all the diodes, is a semiconductor device, which behaves as a bulb lighting up when connected to a voltage generator. The radiated energy can derive experimentally from the characteristic curve of the diode. Therefore, it can be easily seen that each LED is a "non-ohmic conductor", because the voltage-current relation does not follow Ohm's law of direct proportionality.
2 nd Learning Activity (Hypothesis and Experiment Design)
When an electron and a hole meet, they recombine and generate a photon; electrical energy of the couple is transformed in the electromagnetic energy of the photon.
• Discuss with the students about experiment design.
• Identify the tools needed to get the current-voltage curves.
• Building the circuit.
3 rd Learning Activity (Experiment activity)
Measure the threshold voltage for different color LEDs:
• Acquisition of the measures: varying the supply voltage of the circuit the students will measure the function of V diode .
• To plot the trend of the current as a function of the potential difference.
• Fit for the linear part of the curve of the four LEDs we used, I = mV - n. If I = 0 one obtains V threshold .
4 th Learning Activity (Discussion and conclusion)
• The students drawn the characteristic curve of dyed LEDs and using Excel determine the equation of the linear part;
• With the calculation performed with Excel software, the students can determine the experimental value of Planck's constant.
• For each value of h the student calculates the percentage error.
• The light emission of the LED starts from a value of potential (V g ) that corresponds to the voltage for which the electrons are able to recombine with “holes” of the valence band. Experimentally, in order to determine the value of this potential the students proceed with an extrapolation of the linear part of the intensity curve of the current-voltage until it meets the x-axis.
• The value given by the intersection represents the voltage value, which multiplied by the electron charge gives us the amount of the energy of the emitted photons.
• Compare the experimental value of h with that of the literature.
PHOTOELECTRIC EFFECT METHOD
1 st Learning Activity (Developing the concept in order that students can gain an appreciation of the following)
A Hg vapor lamp emits monochromatic light, exploiting the potential of an optical bench, passes through filters of different wavelength and strikes a photo emissive surface K. Also interesting is to note that the incident light is transformed into electricity.
2 nd Learning Activity (Hypotheses and Experimental Design)
The current "emitted" from the photoemission surface can be suitably “braked” up to be stopped by a suitable contrary voltage (potential arrest), capable of preventing the electrons to reach the opposite polarity of the emissive device.
• Discussing with the students on the modality of the of the apparatus structure.
• Finding the best selection and sequence of filters, diaphragms and positioning in the optical bench.
• Developing the experimental apparatus
3 nd Learning Activity (experimental activity)
Measuring the potential stop for the different colored filters:
1. Development of the experience: changing the generator opposite voltage and bringing the index calibration of the micro-ammeter connected to the "0", it can be easily identified with the aid of a milliammeter V a .
2. Tracing the course of the current as a function of the potential difference.
4 nd Learning Activity (discussion and conclusion)
1. Students draw the characteristic curve of the color filters and using Excel determines the equation of the linear part.
2. With the calculation made the students are able to determine the experimental value of Planck's constant.
3. For each value of h the student calculates the percentage of error.
The resetting of the micro ammeter is tied to the value of the potential (V a ) which corresponds to the voltage for which the electrons are able to recombine with "holes” in the valence band. Experimentally, in order to determine the value of this potential, students proceed with a fine calibration of the generator, which is capable to reset the measure of the micro ammeter.
Then the experimental value of h is compared to the one present in the scientific literature.
2. Experimental Design
One of the methods to measure the Planck constant is to derive the voltage-current characteristic curve for a series of different color LEDs.
The method uses the energy emitted when a photon is produced as shown in Figure 1

- PPT PowerPoint Slide
- PNG Larger image(png format)

- NEW View larger figure in new window
- NEXT View next figure

- PREV View previous figure
The diode LED, as all the diodes, is a semiconductor device [ 2 ] , which behaves as a bulb lighting up when connected to a voltage generator. LED’s emit light only when the voltage is forward biased and above a minimum threshold value. Above the threshold, value the current increases exponentially with voltage. The radiated energy can derive experimentally from the characteristic curve of the diode. Therefore, we can observe that each LED is a "non-ohmic conductor", because the voltage-current relation does not follow Ohm's law of direct proportionality.
Figure 2 shows the characteristic curve of a LED. As can be seen from the figure up to a certain voltage value (V<Vg) the LED does not conduct, then for higher voltages (V>Vg) the LED begins to conduct and to emit light. To determine the value of Vg is necessary to make a linear fit of the linear part of the curve and calculate the point where this line intercepts the x-axis. We obtain a value of voltage ( V g ) which, multiplied by the electron charge (e = 1.602x10 -19 C), gives us precisely the energy of the emitted photons ( E g = eV g ).
The equation of the linear part obtained using Excel is:
With the calculation performed with Excel software, we can determine the experimental value of Planck's constant. Starting from
And finally
And setting I = 0
And all the same time
we determine
Using a particular device (photo emissive surface K), the interpretation of the photoelectric effect is based on a very simple and intuitive relation:
where E i is the energy of the incident photon, L the extraction work of the electron from the metal and K e the kinetic energy of the electron released.
This relation, explaining the magnitudes, can be rewritten in the following way:
At this point, by applying a d.d.p. as to have the polarities reversed: cathode → surface photoemission cathode and anode → collector, the electrons are clearly slowed and, acting on the micro ammeter potentiometer, you can go up to block the emitted electron flow (current). This d.d.p. takes the name of potential stop ( V a ), and then Eq. 4 becomes:
If this operation was repeated for two different radiation accidents, the result would be:
and subtracting member to member:
up to obtain:

The apparatus used in this experiment is shown in Figure 3 and is composed by:
• Power supply
• Diode bridge
• LED system
• Two Multimeters: one to be used as voltmeter and the other as an ammeter
The power supply is used to put in operation with the main power supply all equipment that cannot be connected directly to the 220V socket.
The diode bridge is used to convert an AC power signal into a signal in continuous energy.
The circuit that was used to experimentally determine Planck's constant is illustrated below (Figure 4):

The apparatus used in this experiment is shown in Figure 5:

The apparatus used for the measurements consisting of:
1. Optical Bench (Figure 5)
3. Photo emissive Surface
A voltage source, a measuring amplifier and a digital multimeter to use as a micro-ammeter to determine the resetting of the current.
The method represented therefore is based on the photoelectric effect use: an incident electromagnetic radiation, which strikes a metal photoemission surface, if equipped with a particular frequency (energy), can emit electrons to the previously mentioned surface. These electrons can be detected in the form of current. (Figure 6)

The experimental measurements are summarized in Table 1 .
The results using the equations 2 and 3 are summarized in Table 2 ,
Table 1. Measurements of LEDs

- NEW View table in a new window
- NEXT View next table
Here we reported in the graph the linear part for one of the four LEDs: red (Figure 7)
Table 2. Values of h

- PREV View previous table

The equation obtained with Excel is I F = 0.77V F - 1.36.
For I = 0 we obtain
and consequently
A similar behaviour is obtained for all other coloured leds.
The resulting measurements are summarized in Table 3 .
Table 3. Data of Potential Arrest

Table 3 represents the data relating to the different values of the potential shutdown. For each filter, ten measures have been taken. The students, with the help of a spreadsheet, have arranged to iterate the calculation of h as identified by the equation 5. For example, by referring to the two filters, blue and green, the relative potentials of arrest were inserted into the equation above, and by using the tabulated value of their frequencies together with the one of the electron charge, the result is:
Figure 8 presents the graph on the averages course of the arrest potentials measures, related to the specific frequencies of incident radiation.

4. Discussion
For each values obtained of h has been calculated the percentage error as
The experimental value of h is compared to the one present in the scientific literature. In Table 4 , h measured values are confronted.
Table 4. Comparison of the values of h calculated by using the two methods

For what concerns the limits connected to the two methods, it can be easily said that the major problem is related to errors in the potential measurement, which is obtained by extrapolating the linear part of the curve of one LED and the same potential arrest measurement. Since, however, the purpose of the work appears to have definitely educational aspects, what is relevant though, beyond acceptable values, is the order of magnitude of the measures, which is still respected.
5. Conclusions
Within the framework of a master on the Didactics of Science, the authors respectively a chemist and a physicist offer their students two different ways to measure the Planck’s constant: as measurement of the energy of the photons emitted by LEDs diode and the study of the photoelectric effect induced by the light emitted from a mercury lamp.
The authors, involved students by using the IBSE methodology in a series of activities of observation, exploration, construction of circuits, adjustment of a optical bench, use of computer equipment, data processing, analysis of the errors, which allowed them to acquire an experimental mode to calculate the Planck constant.
In the first method, the value of Planck's constant has been determined through current-voltage measurements. The mean value obtained is within the limits of experimental error and in good agreement with the value of literature, considering the available experimental apparatus.
The light emission of the LED starts from a value of potential ( V g ) that corresponds to the voltage for which the electrons are able to recombine with “holes” of the valence band.
In the second method, the mean value is obtained by h single values measured in relation to the confrontation among all the different filters: Blue - Green, Blue – Yellow and Green – Yellow. The arrest potentials used are those of the mean values measured in Table 2 . Even in this case, the mean value obtained is within the limits of experimental error and in good agreement with the value of literature, considering the available experimental apparatus.
Acknowledgements
Andrea Checchetti thanks Giovanni Orlando from I.I.S. “Eleonora Pimentel” of San Giovanni in Fiore (Italy) for his help. Alessandro Fantini thanks prof. Luigi A. Macrì for his contribution.
The authors thanks Dr. Gabriella Fantini (Italy) and prof. Donato Martano for their help throughout the translation.
The Institute of Higher Education “Eleonora Pimentel” of San Giovanni in Fiore (Italy) and the Scientific Lyceum “Enrico Fermi” of Catanzaro Lido (Italy) supported this work.
- Full-Text PDF
- Full-Text ePUB
- Citation-(RIS Format)
- Citation-(BibTeX Format)
- Citation-(EndNote Format)

- Conferences
- Special Issues
- Google Scholar
- VIRAL HEPATITIS CONGRESS
- JournalTOCs
Help & Contacts
- Questionnaire


IMAGES
COMMENTS
By using the equation eV t hf, we can determine Planck’s constant h. This is only approximate, since, as mentioned above, eV t < hf , because some of the energy can be supplied by thermal motion. A better method is therefore to plot V t vs frequency and determine the slope of a straight trendline fitting the data.
To determine Planck’s constant h, we take the slope s from our graph and calculate . using the known value . Alternatively, we can write equation (3) as . calculate h for each LED, and take the average of our results.
Jan 22, 2019 · Planck’s Constant using LED Determination of Planck’s constant by using light-emitting diodes (LEDs). APPARATUS: Planck’s constant kit, wires, graph paper, and 3-4 LEDs. FORMULA USED: Planck’s constant is h = eV /ν, where e is an electronic charge, V is the voltage reading in a voltmeter, and v is the frequency of a particular LED color.
For determination of Planck’s Constant and work function: 1. Insert the red color filter (635nm), set light intensity switch (12) at strong light, voltage direction switch (16) at ‘-‘, display mode switch (10) at current display. 2. Adjust to de-accelerating voltage to 0 V and set current multiplier (4) at X0.001. Increase the de-
Planck’s constant h is a fundamental constant of quantum physics. For example it used to describe the energy of a photon which is smallest unit of energy of light of a given frequency. The photon energy is E= hf =h c λ where h is the Planck’s constant, f and are the frequency and wavelength and c is the speed of light in vacuum.
The Planck constant was introduced by Max Planck back in 1900. The significance of Planck’s constant is that a photons energy (a ‘quanta’ of energy) can be determined by frequency of radiation and Planck’s constant. The Planck constant can be determined with the use of an LED and power source (as well as an Ammeter and Voltmeter for ...
3 The Experiment We employ light emitting diodes (LEDs) to determine Planck’s constant. The basic idea in this measurement is that the photon energy given by E = hν is equal to the energy gap Eg between the valence and conduction bands of the diode. The gap energy Eg is equal to the height of the energy barrier that the electrons have to ...
The aim of this experiment was to utilize the Inquiry Based Science Education, (IBSE) in order to offer the students a better understanding of the light-matter interaction. The inquiry-based teaching addresses the question of how the students make observations, develop hypotheses about phenomena, and devise tests to investigate their hypotheses. Using IBSE they share responsibility within the ...
Planck constant is determined from slop. Table 2 The obtained value of Planck constant from four experiments. No. Planck constant (J.s) 1 6.630 10-34 2 6.630 10-34 3 6.620 10-34 4 6.620 10-34 Average (6.625 ± 0.003) 10-34. The Planck constant value of each experiment and average value are shown in Table 2. The average Planck
A common device, the light emitting diode or LED, could be designed only because some engineers understood quantum science. Thus, knowledge of the value of Planck’s constant is “hidden” in the LED. This short tutorial will show you how you can determine a value for Planck’s Constant by using LEDs.