
- Idiopathic intracranial hypertension
- Report problem with article
- View revision history

Citation, DOI, disclosures and article data
At the time the article was created Paresh K Desai had no recorded disclosures.
At the time the article was last revised Keshaw Kumar had no financial relationships to ineligible companies to disclose.
- Pseudotumour cerebri
- Pseudotumor cerebri
- Benign intracranial hypertension
- Idiopathic intracranial hypertension (IIH)
- Benign intracranial hypertension (BIH)
- Meningitis serosa
Idiopathic intracranial hypertension (IIH) , also known as pseudotumor cerebri , is a syndrome with signs and symptoms of increased intracranial pressure but where a causative mass or hydrocephalus is not identified.
On this page:
Terminology, epidemiology, clinical presentation, radiographic features, treatment and prognosis, history and etymology, differential diagnosis.
- Cases and figures
- Imaging differential diagnosis
The older term benign intracranial hypertension is generally frowned upon due to the fact that some patients with idiopathic intracranial hypertension have a fairly aggressive clinical picture with rapid visual loss.
Interestingly, as it has become evident that at least some patients present with IIH due to identifiable transverse sinus stenosis , some authors now advocate reverting to the older term pseudotumor cerebri as in these patients the condition is possibly not idiopathic 15 . An alternative approach is to move these patients into a group termed secondary intracranial hypertension 15 .
By far the most commonly affected demographic is middle-aged obese females, affected approximately ten times more frequently than other patient groups 34 . The etiological link between being female, overweight and developing idiopathic intracranial hypertension remains to be elucidated.
Obesity is encountered in the majority of cases 34 , and as the prevalence of obesity is increasing, so too is the incidence of this diagnosis 31 .
Less commonly IIH can also be encountered in males, usually older and less likely to be obese 15 . It is rare in the pediatric population, being more common in the 12-17 year age group than in the 2-12 year age group 15,29 .
Associations
A variety of conditions are known to be associated with idiopathic intracranial hypertension including:
adrenal insufficiency
Cushing disease
hypoparathyroidism
hypothyroidism
excessive thyroxine replacement in children
tetracyclines, such as doxycycline 2
growth hormone
hypervitaminosis A from dietary intake or other retinoids, such as all-trans retinoic acid, isotretinoin, or retinol
chronic renal failure
systemic lupus erythematosus (SLE)
Modified Dandy criteria
The modified Dandy criteria were revised to establish the diagnosis in the Idiopathic Intracranial Hypertension Treatment Trial 24 :
presence of signs and symptoms of increased intracranial pressure
absence of localizing findings on neurologic exam except those known to occur from increased intracranial pressure
absence of deformity, displacement, or obstruction of the ventricular system and otherwise normal neurodiagnostic studies, except for evidence of increased CSF pressure*; abnormal neuroimaging except for empty sella turcica, optic nerve sheath with filled out CSF spaces, and smooth-walled non-flow-related venous sinus stenosis or collapse should lead to another diagnosis
awake and alert patient
no other cause of increased intracranial pressure present
*The opening CSF pressure should be either >25.0 cm H 2 O or 20.0-25.0 cm H 2 O with at least one of the following additional findings:
pulse-synchronous tinnitus
abducens nerve palsy
Frisen grade II papilledema
echography negative for drusen or other disc anomalies mimicking disc edema (pseudopapilledema)
lateral sinus stenosis or collapse
partially empty sella and optic nerve sheaths with filled out CSF spaces
Revised Friedman criteria
A competing set of diagnostic criteria were proposed in 2013 and are also commonly used 37 . The original terminology was for pseudotumor cerebri syndrome but the term idiopathic intracranial hypertension has since supplanted it.
The criteria place patients into one of four diagnostic subgroups:
definite idiopathic intracranial hypertension : opening pressure ≥25 cm CSF (H 2 O) and papilledema
probable idiopathic intracranial hypertension: opening pressure <25 cm CSF and papilledema
definite idiopathic intracranial hypertension without papilledema : opening pressure ≥25 cm CSF and abducens nerve palsy
suggested idiopathic intracranial hypertension without papilledema : opening pressure ≥25 cm CSF and ≥3 out of 4 neuroimaging signs ( empty sella , flattening of the posterior aspect of the globe, distension of the perioptic subarachnoid space, or transverse venous sinus stenosis )
These subgroups have several additional common requirements:
neurologic examination: normal except for cranial nerve abnormalities
neuroimaging: no hydrocephalus, mass, structural lesion, or abnormal meningeal enhancement on MRI (without and with contrast) and, if not female or obese, MRV (if MRI is unavailable or contraindicated, contrast-enhanced CT may be used)
laboratory: normal CSF composition
For children, the threshold opening pressure is 28 cm CSF rather than 25 cm CSF, unless the child is not sedated and not obese.
Patients usually present with headaches, visual problems (transient or gradual visual loss), pulse-synchronous tinnitus , photopsia , and/or eye pain 15,31 .
Papilledema is the hallmark finding on fundoscopic examination, which is typically bilateral but uncommonly may be unilateral or even absent, making the clinical diagnosis difficult 6 . Neurological examination is usually normal, except visual field deficit or sixth cranial nerve palsy are sometimes encountered.
Lumbar puncture is central to diagnosis. The CSF composition is normal but the opening pressure is elevated (with 20-25 cm H 2 O considered equivocal and >25 cm H 2 O considered definitely abnormal). It is controversial whether positioning during lumbar puncture is clinically important, with some insisting that lateral decubitus is the most accurate but others believing the default position for fluoroscopy-guided lumbar puncture, prone, is close enough 25 . It should also be noted that opening pressure can vary during the day. One study continuously measuring CSF pressures demonstrated many patients had intermittent pressure waves with amplitudes of 50–80 mmHg (68–109 cm H 2 O) that lasted 5 to 20 minutes 26 .
Aberrant arachnoid granulations , also referred to as meningoceles, can result in secondary CSF leaks that can present as rhinorrhea, otorrhea, intracranial hypotension, and recurrent bacterial meningitis 7,9 . In such patients it is often only after dural repair that intracranial hypertension becomes evident; presumably, the CSF leak from the meningocele normalized pressure 9 .
The pathogenesis is poorly understood. Various mechanisms have been proposed, including decreased CSF absorption, increased CSF production, increased intravascular volume, increased intracranial venous pressure, hormonal changes, altered aquaporin-4 channels and abnormality in function of the glymphatic system 1,15,32-34 .
Venous sinus stenosis is increasingly recognized as an important factor although whether it is the primary inciting abnormality or a potentiating factor remains to be fully established. The increasingly established clinical efficacy of venous stenting suggests that it is, however, not merely a biomarker 31 . It has also been shown that the pressure within the torcula or the dural venous sinuses and the opening pressure measured at lumbar puncture are very closely correlated 31 .
A study also found an association between decreased glymphatic clearance and papilledema in patients with IIH 32 . Whether this is a primary driving cause or also the sequela of impaired venous outflow remains to be determined 32-34 .
Imaging of the brain with CT or MRI without and with contrast, and possibly CT or MR venography, is essential in patients with suspected idiopathic intracranial hypertension to exclude elevated CSF pressure due to other causes such as brain tumor , dural sinus thrombosis , hydrocephalus , etc.
In the absence of a cause for intracranial hypertension, imaging features that support the diagnosis of idiopathic intracranial hypertension include 5,6-9,15,23 :
optic nerve sheath distension (70%, more sensitively/reliably detected by high-resolution 3D T2-weighted imaging) 23,40,41,43
optic nerve sheath diameter >5.3-6 mm or subarachnoid space >2 mm measured 3 mm posterior to the globe on axial or coronal images ref
a small series (c. 2024) showed greater accuracy in predicting IIH using the "arachnoid bulk ratio" but this requires further validation 48
posterior globe flattening (60%)
optic nerve tortuosity in vertical or horizontal planes (40%)
papilledema /optic nerve head protrusion (30%)
optic nerve head enhancement (~ 45%, range10-80%; more sensitively detected by contrast-enhanced 3D T2-weighted FLAIR) 36
enlarged arachnoid outpouchings
partially empty sella turcica (60%)
pituitary occupies less than two-thirds of the pituitary fossa (at least pituitary height loss grade III) 38 but some use a lower threshold of 50% 23
pituitary gland height <4.8 mm 43,44
Meckel cave enlargement 9,18 (but sometimes it is narrowed 39 )
arachnoid pits ( aberrant arachnoid granulations )/small meningoceles , typically within the temporal bone and sphenoid wing 9
enlarged oculomotor cistern (CSF space around the oculomotor nerve in the lateral wall of the cavernous sinus ) 18
prominent perivascular spaces 30
venous outflow obstruction
transverse sinus stenosis (80%) 23,31
significant bilateral transverse sinus narrowing due to any combination of arachnoid granulations (focal, most commonly at the lateral aspect near the transverse-sigmoid sinus junction), extrinsic compression (segmental, which can be relieved after CSF withdrawal 11,12 ), or hypoplasia/aplasia (diffuse), not related to current or remote thrombosis 8
index of transverse sinus stenosis ≥4: the index is the product of stenosis grades on the left and right sides, where 0 = normal, 1 = stenosis up to one-third (<33%) compared to the immediate pre-stenotic segment, 2 = stenosis between one-third and two-thirds (33-66%), 3 = stenosis more than two-thirds (>66%), and 4 = hypoplasia defined as full-length transverse sinus diameter less than one-third of the superior sagittal sinus 5,28,42
combined venous conduit patency score ≤4: the score is the sum of patency grades on the left and right transverse-sigmoid conduit, where 4 = normal (75-100% of the diameter of the distal superior sagittal sinus), 3 = mild stenosis (50-75% patent), 2 = moderate stenosis (25-50% patent), 1 = severe stenosis or hypoplasia (<25% patent), and 0 = absent (discontinuity or aplastic) 45,46
internal jugular vein stenosis (including styloidogenic jugular venous compression ) 47
acquired cerebellar tonsillar ectopia (20%) 16,23
slit-like ventricles (15%) 15,23
increased subcutaneous fat thickness in the scalp and neck (a slim patient is unlikely to develop idiopathic intracranial hypertension) 17
Although bony changes are permanent, the rest may be reversible with treatment 3,11,12 .
It is important to note that some of these findings in isolation may be normal (such as partially empty sella, particularly in older patients). Optimal diagnostic accuracy requires taking into account the entire constellation of multiple imaging findings as well clinical features.
Angiography (DSA)
In addition to enabling venous stenting, catheter venography allows for venous manometry to be performed to evaluate transverse sinus stenosis 31 . Serial measurements of pressure from the superior sagittal sinus down to the internal jugular vein and right atrium allows for the detection of a focal pressure differential across of stenosis (so-called trans-stenosis gradient) 31 .
First-line treatment options include 13,31 :
weight loss in patients with a BMI >30 kg/m 2
weight loss of ~15% is possibly curative
carbonic anhydrase inhibitors
acetazolamide
Invasive treatment options, usually reserved for refractory cases, include 13 :
venous sinus stenting for transverse sinus stenosis
typically reserved for severe cases with a trans-stenotic gradient of >8 mmHg 31
increasingly shown to be effective 4,10,14,31,35
~80% improved headache 14,35 , ~95% improved tinnitus 14,35 , ~90% improved papilledema 14,35 , ~90% improved visual symptoms 35
reduction in opening pressure by ~15 cmH 2 O 35
treatment failure ~13% 14,35
internal jugular venous decompression
less well established
relies on either stenting or removal of compressing structure (e.g. styloidectomy, mastoid process, muscles, masses, etc.) 31
bariatric surgery as a surgical weight loss strategy
optic nerve sheath fenestration (only if vision is acutely threatened)
serial CSF letting or CSF shunting (e.g. ventriculoperitoneal shunt , lumboperitoneal shunt )
Idiopathic intracranial hypertension was first reported in 1893 by Heinrich Quincke, and termed "meningitis serosa". The term "pseudotumor cerebri" was later introduced in 1904, and later still "benign intracranial hypertension" in 1955 (not to be confused with benign intracranial hypotension ) 15 .
Other causes of intracranial hypertension and papilledema should be sought, such as intracranial mass or hydrocephalus. Even without mass effect, venous obstruction (e.g. venous sinus thrombosis ) and leptomeningeal diseases (e.g. meningitis ) can mimic the intracranial findings. Therefore, CT or MR venography and lumbar puncture are part of the usual workup of suspected idiopathic intracranial hypertension.
Additionally, in patients with prominent cerebellar tonsillar ectopia , the possibility that all findings are in fact due to a Chiari I malformation should be considered, particularly as there is substantial overlap in the demographics and clinical presentation of the two patient groups 16,19 . It has even been suggested that some cases of symptomatic intracranial hypertension are secondary to a Chiari I malformation 20 . Importantly, however, every attempt should be made to distinguish between the two entities as treatment is different and symptom relief for patients with idiopathic intracranial hypertension with posterior fossa decompression is insignificant 21 .
Quiz questions
- 1. Silbergleit R, Junck L, Gebarski S, Hatfield M. Idiopathic Intracranial Hypertension (Pseudotumor Cerebri): MR Imaging. Radiology. 1989;170(1 Pt 1):207-9. doi:10.1148/radiology.170.1.2909098 - Pubmed
- 2. Digre K. Not So Benign Intracranial Hypertension. BMJ. 2003;326(7390):613-4. doi:10.1136/bmj.326.7390.613 - Pubmed
- 3. Zagardo M, Cail W, Kelman S, Rothman M. Reversible Empty Sella in Idiopathic Intracranial Hypertension: An Indicator of Successful Therapy? AJNR Am J Neuroradiol. 1996;17(10):1953-6. PMC8337556 - Pubmed
- 4. Higgins J, Cousins C, Owler B, Sarkies N, Pickard J. Idiopathic Intracranial Hypertension: 12 Cases Treated by Venous Sinus Stenting. J Neurol Neurosurg Psychiatry. 2003;74(12):1662-6. doi:10.1136/jnnp.74.12.1662 - Pubmed
- 5. Beier D, Korsbæk J, Bsteh G et al. Magnetic Resonance Imaging Signs of Idiopathic Intracranial Hypertension. JAMA Netw Open. 2024;7(7):e2420138. doi:10.1001/jamanetworkopen.2024.20138 - Pubmed
- 6. Suzuki H, Takanashi J, Kobayashi K, Nagasawa K, Tashima K, Kohno Y. MR Imaging of Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2001;22(1):196-9. PMC7975547 - Pubmed
- 7. Schuknecht B, Simmen D, Briner H, Holzmann D. Nontraumatic Skull Base Defects with Spontaneous CSF Rhinorrhea and Arachnoid Herniation: Imaging Findings and Correlation with Endoscopic Sinus Surgery in 27 Patients. AJNR Am J Neuroradiol. 2008;29(3):542-9. doi:10.3174/ajnr.A0840 - Pubmed
- 8. Leach J, Fortuna R, Jones B, Gaskill-Shipley M. Imaging of Cerebral Venous Thrombosis: Current Techniques, Spectrum of Findings, and Diagnostic Pitfalls. Radiographics. 2006;26 Suppl 1(suppl_1):S19-41; discussion S42-3. doi:10.1148/rg.26si055174 - Pubmed
- 9. Bialer O, Rueda M, Bruce B, Newman N, Biousse V, Saindane A. Meningoceles in Idiopathic Intracranial Hypertension. AJR Am J Roentgenol. 2014;202(3):608-13. doi:10.2214/AJR.13.10874 - Pubmed
- 10. Ahmed R, Wilkinson M, Parker G et al. Transverse Sinus Stenting for Idiopathic Intracranial Hypertension: A Review of 52 Patients and of Model Predictions. AJNR Am J Neuroradiol. 2011;32(8):1408-14. doi:10.3174/ajnr.A2575 - Pubmed
- 11. Rohr A, Dörner L, Stingele R, Buhl R, Alfke K, Jansen O. Reversibility of Venous Sinus Obstruction in Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2007;28(4):656-9. PMC7977370 - Pubmed
- 12. Scoffings D, Pickard J, Higgins J. Resolution of Transverse Sinus Stenoses Immediately After CSF Withdrawal in Idiopathic Intracranial Hypertension. J Neurol Neurosurg Psychiatry. 2007;78(8):911-2. doi:10.1136/jnnp.2006.111765 - Pubmed
- 13. Mollan S, Davies B, Silver N et al. Idiopathic Intracranial Hypertension: Consensus Guidelines on Management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088-100. doi:10.1136/jnnp-2017-317440 - Pubmed
- 14. Nicholson P, Brinjikji W, Radovanovic I et al. Venous Sinus Stenting for Idiopathic Intracranial Hypertension: A Systematic Review and Meta-Analysis. J Neurointerv Surg. 2019;11(4):380-5. doi:10.1136/neurintsurg-2018-014172 - Pubmed
- 15. Degnan A & Levy L. Pseudotumor Cerebri: Brief Review of Clinical Syndrome and Imaging Findings. AJNR Am J Neuroradiol. 2011;32(11):1986-93. doi:10.3174/ajnr.A2404 - Pubmed
- 16. Aiken A, Hoots J, Saindane A, Hudgins P. Incidence of Cerebellar Tonsillar Ectopia in Idiopathic Intracranial Hypertension: A Mimic of the Chiari I Malformation. AJNR Am J Neuroradiol. 2012;33(10):1901-6. doi:10.3174/ajnr.A3068 - Pubmed
- 17. Saindane A, Lim P, Aiken A, Chen Z, Hudgins P. Factors Determining the Clinical Significance of an "Empty" Sella Turcica. AJR Am J Roentgenol. 2013;200(5):1125-31. doi:10.2214/AJR.12.9013 - Pubmed
- 18. San Millán D & Kohler R. Enlarged CSF Spaces in Pseudotumor Cerebri. AJR Am J Roentgenol. 2014;203(4):W457-8. doi:10.2214/AJR.14.12787 - Pubmed
- 19. Bejjani G. Association of the Adult Chiari Malformation and Idiopathic Intracranial Hypertension: More Than a Coincidence. Med Hypotheses. 2003;60(6):859-63. doi:10.1016/s0306-9877(03)00064-1 - Pubmed
- 20. Fukuoka T, Nishimura Y, Hara M et al. Chiari Type 1 Malformation-Induced Intracranial Hypertension with Diffuse Brain Edema Treated with Foramen Magnum Decompression: A Case Report. NMC Case Rep J. 2017;4(4):115-20. doi:10.2176/nmccrj.cr.2016-0278 - Pubmed
- 21. Alnemari A, Mansour T, Gregory S, Miller W, Buehler M, Gaudin D. Chiari I Malformation with Underlying Pseudotumor Cerebri: Poor Symptom Relief Following Posterior Decompression Surgery. Int J Surg Case Rep. 2017;38:136-41. doi:10.1016/j.ijscr.2017.07.039 - Pubmed
- 22. Suzuki H, Takanashi J, Kobayashi K, Nagasawa K, Tashima K, Kohno Y. MR Imaging of Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2001;22(1):196-9. PMC7975547 - Pubmed
- 23. Kwee R & Kwee T. Systematic Review and Meta-Analysis of MRI Signs for Diagnosis of Idiopathic Intracranial Hypertension. Eur J Radiol. 2019;116:106-15. doi:10.1016/j.ejrad.2019.04.023 - Pubmed
- 24. Wall M, Kupersmith M, Kieburtz K et al. The Idiopathic Intracranial Hypertension Treatment Trial: Clinical Profile at Baseline. JAMA Neurol. 2014;71(6):693-701. doi:10.1001/jamaneurol.2014.133 - Pubmed
- 25. Lueck C & McClelland C. Is Positioning During Lumbar Puncture Clinically Significant? J Neuroophthalmol. 2019;39(2):268-72. doi:10.1097/wno.0000000000000696 - Pubmed
- 26. Johnston I & Paterson A. Benign Intracranial Hypertension. Brain. 1974;97(1):301-12. doi:10.1093/brain/97.1.301 - Pubmed
- 27. Iencean S. Idiopathic Intracranial Hypertension and Idiopathic Normal Pressure Hydrocephalus: Diseases with Opposite Pathogenesis? Med Hypotheses. 2003;61(5-6):526-8. doi:10.1016/s0306-9877(03)00208-1
- 28. Carvalho G, Matas S, Idagawa M et al. A New Index for the Assessment of Transverse Sinus Stenosis for Diagnosing Idiopathic Intracranial Hypertension. J NeuroIntervent Surg. 2016;9(2):173-7. doi:10.1136/neurintsurg-2016-012605 - Pubmed
- 29. Malem A, Sheth T, Muthusamy B. Paediatric Idiopathic Intracranial Hypertension (IIH)-A Review. Life (Basel). 2021;11(7):632. doi:10.3390/life11070632 - Pubmed
- 30. Jones O, Cutsforth-Gregory J, Chen J, Bhatti M, Huston J, Brinjikji W. Idiopathic Intracranial Hypertension is Associated with a Higher Burden of Visible Cerebral Perivascular Spaces: The Glymphatic Connection. AJNR Am J Neuroradiol. 2021;42(12):2160-4. doi:10.3174/ajnr.A7326 - Pubmed
- 31. Fargen K, Coffman S, Torosian T, Brinjikji W, Nye B, Hui F. "Idiopathic" Intracranial Hypertension: An Update from Neurointerventional Research for Clinicians. Cephalalgia. 2023;43(4):3331024231161323. doi:10.1177/03331024231161323 - Pubmed
- 32. Schartz D, Finkelstein A, Hoang N, Bender M, Schifitto G, Zhong J. Diffusion-Weighted Imaging Reveals Impaired Glymphatic Clearance in Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2024;45(2):149-54. doi:10.3174/ajnr.A8088 - Pubmed
- 33. Lenck S, Radovanovic I, Nicholson P, Hodaie M, Krings T, Mendes-Pereira V. Idiopathic Intracranial Hypertension: The Veno Glymphatic Connections. Neurology. 2018;91(11):515-22. doi:10.1212/WNL.0000000000006166 - Pubmed
- 34. Bezerra M, Ferreira A, de Oliveira-Souza R. Pseudotumor Cerebri and Glymphatic Dysfunction. Front Neurol. 2018;8:734. doi:10.3389/fneur.2017.00734 - Pubmed
- 35. Azzam A, Mortezaei A, Morsy M et al. Venous Sinus Stenting for Idiopathic Intracranial Hypertension: An Updated Meta-Analysis. J Neurol Sci. 2024;459:122948. doi:10.1016/j.jns.2024.122948 - Pubmed
- 36. Golden E, Krivochenitser R, Mathews N et al. Contrast-Enhanced 3D-FLAIR Imaging of the Optic Nerve and Optic Nerve Head: Novel Neuroimaging Findings of Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2019;40(2):334-9. doi:10.3174/ajnr.a5937 - Pubmed
- 37. Friedman D, Liu G, Digre K. Revised Diagnostic Criteria for the Pseudotumor Cerebri Syndrome in Adults and Children. Neurology. 2013;81(13):1159-65. doi:10.1212/wnl.0b013e3182a55f17 - Pubmed
- 38. Yuh W, Zhu M, Taoka T et al. MR Imaging of Pituitary Morphology in Idiopathic Intracranial Hypertension. J Magn Reson Imaging. 2000;12(6):808-13. 3.0.co;2-n">doi:10.1002/1522-2586(200012)12:6<808::aid-jmri3>3.0.co;2-n - Pubmed
- 39. Degnan A & Levy L. Narrowing of Meckel's Cave and Cavernous Sinus and Enlargement of the Optic Nerve Sheath in Pseudotumor Cerebri. J Comput Assist Tomogr. 2011;35(2):308-12. doi:10.1097/rct.0b013e31820d7a70 - Pubmed
- 40. Abdelrahman A & Barakat M. MRI Measurement of Optic Nerve Sheath Diameter Using 3D Driven Equilibrium Sequence as a Non-Invasive Tool for the Diagnosis of Idiopathic Intracranial Hypertension. Egypt J Radiol Nucl Med. 2020;51(1):1-7. doi:10.1186/s43055-020-0149-x
- 41. Sotoudeh H, Bowerson M, Parsons M et al. Effect of Spatial Resolution of T2-Weighted Imaging on Diagnostic Efficacy of MRI in Detection of Papilledema. AJR Am J Roentgenol. 2015;204(3):602-7. doi:10.2214/ajr.14.12662 - Pubmed
- 42. Korsbæk J, Jensen R, Høgedal L, Molander L, Hagen S, Beier D. Diagnosis of Idiopathic Intracranial Hypertension: A Proposal for Evidence-Based Diagnostic Criteria. Cephalalgia. 2023;43(3):033310242311527. doi:10.1177/03331024231152795 - Pubmed
- 43. Mallery R, Rehmani O, Woo J et al. Utility of Magnetic Resonance Imaging Features for Improving the Diagnosis of Idiopathic Intracranial Hypertension Without Papilledema. J Neuroophthalmol. 2019;39(3):299-307. doi:10.1097/wno.0000000000000767 - Pubmed
- 44. Hoffmann J, Huppertz H, Schmidt C et al. Morphometric and Volumetric MRI Changes in Idiopathic Intracranial Hypertension. Cephalalgia. 2013;33(13):1075-84. doi:10.1177/0333102413484095 - Pubmed
- 45. Farb R, Vanek I, Scott J et al. Idiopathic Intracranial Hypertension: The Prevalence and Morphology of Sinovenous Stenosis. Neurology. 2003;60(9):1418-24. doi:10.1212/01.wnl.0000066683.34093.e2 - Pubmed
- 46. Morris P, Black D, Port J, Campeau N. Transverse Sinus Stenosis Is the Most Sensitive MR Imaging Correlate of Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2017;38(3):471-7. doi:10.3174/ajnr.a5055 - Pubmed
- 47. Zhou D, Meng R, Zhang X et al. Intracranial Hypertension Induced by Internal Jugular Vein Stenosis Can Be Resolved by Stenting. Euro J of Neurology. 2017;25(2):365. doi:10.1111/ene.13512 - Pubmed
- 48. Berhanu D, Carneiro I, Antunes A et al. Dimensions of Arachnoid Bulk Ratio: A Superior Optic Nerve Sheath Index for Intracranial Pressure. Radiology. 2024;312(1):e240114. doi:10.1148/radiol.240114 - Pubmed
Incoming Links
- CSF otorrhoea
- Chiari I malformation
- Non-pulsatile tinnitus
- Medical abbreviations and acronyms (I)
- Systemic lupus erythematosus (CNS manifestations)
- Papilloedema
- Neurobrucellosis
- Pituitary fossa
- Combined conduit score of sinovenous stenosis
- Idiopathic intracranial hypertension (mnemonic)
- Spaceflight-induced cerebral changes
- Investigating diplopia (summary)
- Pulsatile tinnitus
- Lumboperitoneal shunt
- Optic disc drusen
- Sigmoid sinus diverticulum
- Encephalocele
- Foster Kennedy syndrome
- Cerebellar tonsillar ectopia
- Glymphatic pathway
- Empty sella
- Idiopathic intracranial hypertension - dural venous stenosis
- Bilateral temporal encephaloceles
- Idiopathic intracranial hypertension (IIH) with VI CN palsy - paediatric
- Middle cranial fossa meningoencephalocele
- Idiopathic intracranial hypertension with VI CN palsy - paediatric
- Compressive arachnoid cyst with secondary intracranial hypertension
- Idiopathic Intracranial hypertension with CSF rhinorrhoea
- Question 3049
- Question 3048
- Question 3047
- Question 2997
- Question 2996
- Question 2045
- Question 822
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Editorial Board
- Radiopaedia Team

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Intracranial hypertension.
Sandeep Sharma ; Muhammad F. Hashmi ; Caroline L. Davidson ; Anil Kumar .
Affiliations
Last Update: March 3, 2024 .
- Continuing Education Activity
Intracranial hypertension is a state of pressure elevation within the skull that may cause various neurological disorders. The condition may arise from congenital and acquired etiologies and manifest with a diverse range of symptoms, from mild headaches and nausea to sensory disturbances, seizures, cardiovascular instability, and unconsciousness. Diagnostic and management approaches differ based on the underlying condition. Understanding the mechanisms underlying intracranial pressure alterations is crucial to diagnosis. A deep grasp of evidence-based interventions can help health providers determine the appropriate management strategy for a patient with intracranial hypertension.
This activity for healthcare professionals is designed to sharpen learners' proficiency in evaluating and managing intracranial hypertension. Participants gain a heightened capacity to navigate intracranial hypertension's complexities, informed by evidence-based practices, and a strengthened ability to improve neurological outcomes. Increased diagnostic and management acumen equips learners to collaborate effectively within an interprofessional team caring for patients with intracranial hypertension.
- Identify the signs and symptoms indicative of intracranial hypertension.
- Create a clinically guided diagnostic plan for a patient with possible intracranial hypertension.
- Develop management strategies for patients diagnosed with intracranial hypertension.
- Implement effective coordination and communication strategies within an interprofessional team caring for patients with intracranial hypertension.
- Introduction
Intracranial hypertension is a condition characterized by elevated pressure within the skull. The increase in pressure can exert significant stress on the brain and other intracranial structures, potentially leading to a range of neurological symptoms and complications. Intracranial hypertension's clinical manifestations vary depending on the underlying cause, severity of pressure elevation, and individual patient factors. Common symptoms may include severe headaches, visual disturbances, nausea, vomiting, tinnitus, and, in severe cases, seizures or coma.
Diagnosing intracranial hypertension typically involves a combination of clinical evaluation, results of neuroimaging studies like computed tomography (CT) and magnetic resonance imaging (MRI), and intracranial pressure (ICP) measurement via invasive or noninvasive techniques. Early recognition and prompt management of this condition are essential to prevent potential complications, including permanent neurological damage and even death. Managing ICH typically involves addressing the underlying cause, optimizing cerebral perfusion, and sometimes surgical interventions to relieve pressure on the brain.
Cerebrospinal Fluid and Intracranial Pressure
The human skull has a fixed volume of approximately 1400 to 1700 mL. Intracranial content volume comprises 80% brain parenchyma, 10% cerebrospinal fluid, and 10% blood.
The choroid plexus is the main CSF producer and regulator, secreting around 20 mL per hour, averaging 450 mL per day. Arachnoid granulations reabsorb CSF and drain it into the venous system at similar rates. Normal cerebrospinal fluid (CSF) production varies by age, with typically high production during infancy that declines and stabilizes in childhood and adulthood. CSF pressures greater than 250 mm H20 in adults and 200 mm H20 in children generally signify increased ICP. [1] [2] [3] [4]
Intracranial volume is more or less constant once the sutures completely ossify. Intracranial tissue or fluid volume elevation can raise intracranial pressure, which can occur in the presence of intracranial masses, ventricular stenosis, and hematomas. A large part of treating intracranial hypertension involves mitigating the risk of increased ICP and making timely clinical decisions to prevent adverse consequences.
The brain parenchyma's physiologic volume is relatively constant in adults but may change due to mass lesions or cerebral edema. Cerebral edema can occur following acute hypoxic encephalopathy, sizeable cerebral infarctions, and severe traumatic brain injury (TBI). CSF and blood volume in the intracranial space vary regularly as these are the primary ICP regulators.
Neurological injuries such as stroke or trauma can damage the control mechanisms, maintaining appropriate CSF volumes. Increased CSF production, as in the presence of a choroid plexus papilloma, can make the CSF secretion rate exceed the reabsorption rate. Impaired CSF reabsorption, seen in arachnoid granulation adhesions after bacterial meningitis, can also raise the ICP. CSF drainage interruption and hydrocephalus may arise from conditions such as intraventricular masses, congenital aqueductal stenosis, and acute intraventricular hemorrhage.
Cerebral blood flow (CBF) is the primary intracranial blood volume regulator. Diseases obstructing venous outflow, such as venous sinus thrombosis, jugular vein compression, and neck surgery-associated structural changes, may cause intracranial blood congestion and intracranial hypertension. Idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri, is a term for chronic ICP increase due to unknown causes with no known structural change. [5] [6]
Intracranial hypertension may be divided into primary and secondary causes, some of which are listed below.
Primary or Intracranial Causes
- Trauma, producing epidural hematoma, subdural hematoma, intracerebral hemorrhage, subarachnoid hemorrhage, or contusions
- Brain tumors
- Ischemic stroke
- Nontraumatic intracerebral hemorrhage from aneurysm rupture or hypertensive hemorrhage
- Idiopathic or benign intracranial hypertension
- Hydrocephalus
- Congenital malformations, including aqueductal stenosis, Dandy-Walker malformation, and Chiari malformation
Secondary or Extracranial Causes
- Hypoventilation, causing hypoxia or hypercarbia
- Hypertension
- Airway obstruction
- Metabolic, usually drug-related
- Hyperpyrexia
- High-altitude cerebral edema
- Cervical structural venous outflow obstruction
- Polypharmacy
- Epidemiology
Intracranial hypertension epidemiology depends on the etiology. Conditions presenting with acute ICP elevation are distributed in the population differently from pathologies causing chronic ICP increase. For example, about 60% of spontaneous hemorrhages arise from intracranial bleeding secondary to systemic hypertension. [7] Up to a third of hypertensive hemorrhages occur in patients aged over 80. Amyloid angiopathy is another common etiology of spontaneous intracranial hemorrhage, more common in older patients' cerebral cortices. [8] Subarachnoid hemorrhage occurs with an annual incidence of up to 91 cases out of 100,000, 85% of which are due to aneurysmal rupture. [9] In 2019, 27 million new cases of TBI occurred worldwide, ranging from mild to severe. [10]
Meanwhile, up to 90% of individuals with chronic IIH are women of childbearing age. People with chronic hypertension or obesity have an increased risk of developing intracranial hypertension. The occurrence frequency is 1.0 in 100,000 in the general population, 1.6 to 3.5 in 100,000 among women, and 7.9 to 20 in 100,000 among women who are overweight.
- Pathophysiology
The total volume within the intracranial and spinal canals remains constant with only minute fluctuations. A volumetric increase can elevate ICP. [11] Normal ICP in adults ranges from 10 to 20 cm H20. ICP elevation increases the risk of neural injury from direct compression or CBF reduction. Clinically, CBF is determined indirectly from the measurable parameters, cerebral perfusion pressure (CPP) and mean arterial pressure (MAP), based on the following equation:
Cerebral perfusion pressure (CPP) = Mean arterial pressure (MAP) - Intracranial pressure (ICP)
Inflow Dynamics
CPP is the blood flow pressure to the brain, the force driving oxygen delivery necessary for neuronal functioning. This value remains within the 50 to 100 mm Hg range due to autoregulation. ICP elevation reduces CPP, diminishing blood flow pressure to the brain. The physiologic autoregulatory response to reduced CPP is to increase the MAP systemically and dilate cerebral blood vessels. Consequently, cerebral blood volume rises, further increasing ICP.
However, CPP drops paradoxically, producing a feedback cycle that reduces cerebral flow and perfusion. This feedback loop can cause cerebral ischemia and brain infarction with neuronal death. [12] In intracranial hemorrhage cases, increased blood pressure may worsen intracranial bleeding. A minimum CPP of 60 mm Hg is recommended to maintain adequate cerebral perfusion.
Outflow Dynamics
Continuous CSF and blood venous drainage also regulate total intracranial volume. Acute ventricular CSF flow obstruction without decreasing the choroid plexus' production rate results in CSF accumulation. Increased CSF volume elevates ventricular wall pressure, leading to transependymal flow and a potentially rapidly fatal ICP rise. [13] Chronic CSF reabsorption reduction can occur secondary to ventricular wall changes following pathologies like meningitis and intraventricular hemorrhage. Conditions like acute thrombosis, traumatic occlusion from epidural hematoma, depressed skull fracture, and chronic stenosis can obstruct venous drainage pathways. Outflow occlusion produces intracranial intravascular volume elevation with subsequent ICP elevation. [14]
- History and Physical
Patients with intracranial hypertension may present unconscious, apneic, and pulseless, which are signs of cardiorespiratory arrest. Resuscitative measures must be started immediately for all patients in cardiorespiratory arrest, regardless of cause. A primary survey must be performed promptly to address airway, breathing, and circulatory problems. A more thorough investigation may be pursued once the patient is stable.
History provides valuable insights into the onset, progression, and nature of intracranial hypertension symptoms. The most commonly reported manifestations include headaches, visual changes, nausea, and vomiting. Additional symptoms such as cranial nerve palsies and mental status changes further underscore the condition's neurological impact.
A thorough review of past medical history, including comorbid conditions and medication use, provides essential context for understanding the underlying etiology of intracranial hypertension. Conditions such as hypertension, obesity, thyroid disorders, and prior head trauma may predispose individuals to elevated ICP. Medications with potential neurotoxic effects or those associated with fluid retention warrant consideration as possible contributors to intracranial hypertension. Specific cases are considered below.
Idiopathic Intracranial Hypertension
Chronic ICP elevation often presents as nonspecific headaches likely mediated by the trigeminal nerve's dura and blood vessel pain fibers. Pain is generally diffuse and worse in the mornings or after a Valsalva maneuver. Nausea and vomiting are also commonly reported.
The 2 most frequent IIH symptoms are chronic headache and progressive visual deterioration secondary to papilledema. About 20% to 40% of patients have double vision, most frequently with horizontal diplopia associated with abducens nerve compression and palsy. [15] Transient visual abnormalities occur frequently, often described as a gradual dimming in 1 or both eyes. Visual abnormalities worsen with postural changes. Peripheral visual loss may be reported, most commonly beginning in the nasal inferior quadrant with subsequent central visual field loss. Visual acuity alterations with blurring or distortion may occur.
Variable degrees of loss of color distinction may be reported. Visual loss is permanent in up to 40% of cases after treatment. [16] In more severe or chronic cases, a sudden visual loss can occur due to intraocular hemorrhage. Tinnitus with a pulsing rhythm can occur, exacerbated by supine or bending positions and Valsalva maneuvers. Neurological findings are indications of severe disease.
Acute Intracranial Hypertension
Acute ICP elevation is most often due to traumatic injury, giving rise to mechanical parenchymal or anoxic injury and subsequent cytotoxic edema. Other possible etiologies include intraparenchymal hemorrhage, subdural hematoma, epidural hematoma, or hydrocephalus secondary to acute obstruction or subarachnoid hemorrhage. Initial symptoms of acute ICP elevation include nausea, vomiting, lethargy, confusion, and sometimes irritability. These symptoms' underlying causes can be challenging to identify in the complex inpatient setting with many other metabolic, psychological, and systemic pathologic cofactors.
Brain herniation can occur, producing decreased consciousness or responsiveness. The sites most vulnerable to herniation include the cerebral surface and central transtentorial, uncal, transtentorial, cerebellar tonsillar or foramen magnum, and transcalvarial routes. Focal neurological symptoms depend on the location of pressure-induced irritation and injury. Unconsciousness results from pressure on the midbrain's reticular formations. Respiratory drive and effort changes may occur, leading to respiration and oxygenation failure.
Physical Examination
Physical examination complements the history by providing objective findings indicative of intracranial hypertension. Fundoscopic examination assesses for papilledema, a hallmark sign of elevated ICP. The characteristic findings include optical disc swelling, disc margin blurring, and venous congestion. Cranial nerve examination evaluates for abnormalities in visual acuity, pupillary reactions, facial symmetry, and hearing, providing further insights into neurological function. Motor and sensory assessments aid in identifying focal neurological deficits, while gait and coordination evaluations assess cerebellar function and balance.
A mental status change or depressed sensorium should be promptly evaluated. A complete neurological assessment is essential whenever intracranial hypertension is suspected. Cranial nerve assessment is particularly important for identifying lesions. Pupillary reflex blunting with fixed dilation of one pupil and "down and out" ocular position are highly indicative of pressure irritation or ipsilateral oculomotor nerve injury. [17] Spontaneous periorbital bruising may also be present. Cushing triad classically presents with bradycardia, respiratory depression, and hypertension and is highly indicative of intracranial hypertension.
Infants can have widening cranial sutures and bulging fontanelles. Infants do not display papilledema when ICP is elevated on ocular examination due to the fontanelle's compliance features.
Diagnostic testing for intracranial hypertension aims to confirm ICP elevation and identify potential underlying causes. The diagnostic workup typically involves a combination of imaging studies, lumbar puncture, and ophthalmologic evaluation.
Neuroimaging studies assess brain anatomy, identify structural abnormalities, and detect ICP elevation signs. Common imaging modalities include CT and MRI.
CT scans can identify acute conditions, such as hemorrhage, tumors, or hydrocephalus (see Image . Communicating Hydrocephalus Computed Tomography). CT can also detect ventricular enlargement or cerebral sulci effacement. CT venography assesses venous sinus patency.
MRI allows for superior soft tissue contrast and is particularly valuable for evaluating subtle structural abnormalities, such as small tumors or Chiari malformations, that may contribute to intracranial hypertension. MRI can also detect abnormalities in CSF flow dynamics and assess for complications such as venous sinus thrombosis. MR venography evaluates venous sinus patency and identifies abnormalities such as stenosis or thrombosis, which can contribute to intracranial hypertension.
A lumbar puncture measures ICP directly and assesses CSF composition. An elevated opening pressure (>20 mm Hg in adults) suggests intracranial hypertension. CSF analysis may reveal abnormally increased protein levels or evidence of underlying etiologies such as infection or inflammation.
Additional studies may be conducted based on clinical suspicion. Cerebral angiography evaluates for vascular abnormalities, such as arteriovenous malformations or dural arteriovenous fistulas. CSF flow studies evaluate CSF dynamics and identify CSF circulation abnormalities. Endocrine tests screen for hormonal disorders, such as hypothyroidism or adrenal insufficiency, which can contribute to intracranial hypertension.
Correctly identifying the etiology of intracranial hypertension depends on the combination of a good clinical evaluation and diagnostic testing. Approaches to specific intracranial hypertension cases are explained below.
A brain MRI, with and without contrast, provides a detailed intracranial view when evaluating chronic causes of intracranial hypertension (see Image . Idiopathic Intracranial Hypertension MRI). A lumbar puncture is recommended for diagnosis, allowing for measuring opening pressures and evaluating infectious and inflammatory etiologies. However, an intracranial mass should be ruled out via imaging before performing this procedure to avoid the risk of downward herniation. Invasive ICP measurement may be considered if papilledema evaluation yields negative results despite ongoing clinical suspicion.
Progressive visual deterioration is a common papilledema complication. Thus, a neuro-ophthalmology referral is recommended to examine the visual field in detail and monitor symptoms (see Image . Idiopathic Intracranial Hypertension Fundus Examination). Symptom progression is a strong consideration for procedural intervention. [18]
The initial evaluation of acute intracranial hypertension should include a head CT. Cerebral edema-associated CT scan findings indicating intracranial hypertension include compressed basal cisterns, herniation patterns, cortical sulcal effacement, and midline shift. However, the absence of these findings does not rule out the condition. Complete blood count and metabolic panel are usually checked in all patients with suspected intracranial hypertension to evaluate for infection, anemia, and electrolyte abnormalities. A thorough neurologic examination, followed by serial examinations during management, is critical in evaluating patients with suspected acute ICP elevation.
A ventriculostomy catheter is the preferred ICP monitoring device and may be used for therapeutic CSF drainage to lower ICP. When ventricles cannot be cannulated, intraparenchymal devices using microsensors and fibreoptic transducers may be used. Subdural and epidural monitors are not as accurate as ventriculostomy and parenchymal monitors. [19] [20] [21] [22]
- Treatment / Management
Management of Acute Intracranial Hypertension
A sudden significant ICP increase is a neurosurgical emergency requiring close intensive care unit (ICU) monitoring. The initial management goals are airway protection, hemodynamic stability, and progression arrest. The heart rate, blood pressure, body temperature, ventilation and oxygenation, blood glucose, input and output, and cardiac rhythms must be closely monitored. Patients with suspected intracranial hypertension, especially individuals with severe TBI, should also have ICP monitoring. [23] [24] [25]
Intracranial hypertension management has 4 tiers. Implementation should begin at Tier 0 and progress stepwise if the patient does not improve.
- Elevate the head of the bed to 30° with a neutral position to minimize venous outflow resistance and maximize CSF flow dynamics.
- If a cervical collar is in place for possible cervical spine injuries, ensure the collar is loosened to prevent venous outflow obstruction.
- Hypoxia and hypercapnia can increase ICP, making respiratory management crucial. A normal carbon dioxide partial pressure (PaCO2 = 35-45 mm Hg) and adequate oxygenation (PaO2 > 94%) should be maintained by control ventilation without increasing the PEEP.
- Agitation and pain can increase blood pressure and ICP. Adequate sedation and analgesia are thus necessary adjunctive treatments. Medications with a minimal hypotensive effect are preferred. Hypovolemia can precipitate these medications' hypotensive side effects and should be treated before administration. Shorter-acting agents may allow for interrupting sedation to evaluate neurological status.
- Fever can increase the brain's metabolic rate and is a potent vasodilator. Higher body temperatures can increase cerebral blood flow and ICP. Fever should be controlled with antipyretics and cooling blankets. Infectious causes must be ruled out.
- Blood pressure elevation is commonly seen in patients with intracranial hypertension, especially due to TBI. Higher blood pressures also maintain cerebral perfusion in patients with untreated intracranial mass lesions. Systemic hypertension in these patients may be allowed, in some cases, to optimize cerebral perfusion without compromising overall cardiovascular status. Meanwhile, treating systemic hypertension in the absence of an intracranial mass lesion requires an individualized approach.
- The preferred antihypertensive drugs when managing intracranial pressure include β-blockers, such as labetalol and esmolol, and calcium channel blockers—agents that reduce blood pressure without affecting the ICP. Short-acting agents are favored. However, sodium nitroprusside, nitroglycerin, and nifedipine are generally avoided due to their potential to decrease systemic vascular resistance further and cause cerebral vasodilation.
- Seizures can complicate intracranial hypertension and should be prevented by prophylactic medications, especially in severe TBI.
- Hyperosmolar therapy may be initiated to decrease cerebral edema. Serum sodium concentration elevation induces osmotic fluid diffusion from the cerebral parenchyma into the serum. Baseline sodium levels should be monitored every 4 to 6 hours during therapy. Hypertonic saline can be given as a bolus of 3%, 7%, or 23.4% or as a continuous infusion of 3% saline if a more progressive increase in sodium level is desired.
- Mannitol is commonly used as a hyperosmolar agent, usually given as a bolus of 0.25 to 1 g/kg body weight. However, serum osmolality should be kept below 320 mOsm to avoid renal failure, hypokalemia, and hypoosmolarity.
- An external ventricular catheter may be placed for ICP monitoring and lowering by CSF drainage. In cases where ICP elevation is due to a CSF obstructive pathology, external ventricular drainage is the primary therapeutic method. External ventricular drainage may also be used as needed in diffuse cerebral edema cases for close pressure measurement and CSF drainage.
- Tier 2:
- Temporary hyperventilation with a goal PaCO2 of 30 to 35 mm Hg may be administered, though only up to 24 hours. More extended treatment periods have no benefit and may even be deleterious.
- Barbiturate therapy may be initiated to suppress electroencephalogram bursts and intracranial stimulation. Patients must be on continuous electroencephalogram when administering this treatment, and a general neurology service or neurointensive clinician must be consulted.
- Emergent surgical management should be considered if intracranial hypertension is refractory to medical management.
- Hypothermia may also be induced as part of management to reduce brain activity.
Surgical interventions
Intracranial mass lesions producing elevated ICP should be removed as soon as possible. CSF drainage reduces intracranial volume and pressure immediately. This modality may be used as an adjunct treatment for lowering ICP. However, CSF drainage has limited utility when the brain is diffusely swollen, and the ventricles are collapsed.
Decompressive craniectomy is used for treating severe uncontrolled intracranial hypertension. This procedure surgically removes part of the calvaria to create a window in the skull, allowing the swollen brain to herniate through the bone window, thus relieving pressure.
Management of Idiopathic Intracranial Hypertension
IIH management focuses on alleviating suspected CSF or venous outflow pathologic dynamics. Primary interventions are determined based on the severity of symptoms observed on initial evaluation.
Patients with BMI >30 kg/m² should be counseled on weight loss on initial evaluation, as weight loss of 15% can lead to IIH remission. [18] Acetazolamide therapy may be initiated to decrease CSF production volume if the patient is not at risk of immediate vision loss. The starting dose is 250 to 500 mg/day, titrated to a maximum of 4 g/day, though 1 g/day is generally tolerated. Topiramate at 5 to 50 mg BID is another option to reduce CSF production. However, women should be counseled that the medication may decrease anticontraceptive medications' effectiveness.
Surgical interventions are typically reserved for patients at risk of vision loss. A high-volume lumbar puncture can be performed to decrease ocular pressures and temporize until a more definitive surgical intervention can be achieved. Possible surgical interventions include ventriculoperitoneal shunt and venous sinus stenting. Lateral ventricle volumes in patients with IIH are often small, and intraoperative image guidance can assist in accurate placement.
A 2015 meta-analysis of patients with refractory IIH undergoing CSF diversion demonstrated headache improvement in 80% of patients after CSF diversion, papilledema improvement in 70%, and visual acuity in 45%. Headache improvement was noted in 83% of patients, papilledema improved in 97%, and visual symptoms improved in 78%. [26]
Ventriculoperitoneal shunting complications most commonly present as shunt malfunction (proximal or distal obstruction) requiring shunt revision procedures. Venous sinus stenting is indicated only if dural venous stenosis is observed in vascular imaging studies.
Optin nerve sheath fenestration is reserved for patients with refractory papilledema, which is often asymmetric and has visual symptoms as the primary complaint. Less improvement in headaches was reported following this procedure, though an overall lower procedural complication rate than CSF diversion was documented.
- Differential Diagnosis
Intracranial hypertension can arise from various causes. The conditions below are frequently encountered and must be considered in the differential diagnosis. A thorough clinical assessment and judicious diagnostic evaluation can help differentiate these conditions.
- Acute nerve injury
- Benign intracranial hypertension (Pseudotumor cerebri)
- Cerebrovascular ischemia or hemorrhage
- Intracranial epidural abscess
- Intracranial hemorrhage
- Leptomeningeal carcinoma
- Low-grade astrocytoma
- Lyme disease
- Migraine headache
- Papilledema
- Subarachnoid hemorrhage
- Venous sinus thrombosis
Depending on the etiology, prognosis is highly variable, ranging from lethal to benign. Children usually can tolerate higher ICP for a more extended period.
IIH is not associated with any specific mortality risk, but surgical treatments influence morbidity and mortality. The prognosis of this condition depends on visual function. Untreated disc edema can cause irreversible optic neuropathy and loss of color vision.
Short-lived acute intracranial hypertension has a good prognosis when treated promptly. However, treatment delays and the presence of a malignant etiology are associated with a poor prognosis. Many patients who survive develop permanent neurological deficits. [27] [28]
- Complications
Complications of intracranial hypertension vary, depending on the underlying etiology. These complications include:
- Optic neuropathy
- Loss of vision
- Respiratory arrest
Patients with preexisting conditions must be counseled to seek medical attention promptly if symptoms appear and persist despite appropriate initial treatment.
- Consultations
Evaluation and management of intracranial hypertension often require interdisciplinary collaboration. The interprofessional approach optimizes outcomes for patients with this condition. The following specialties are involved in the care of patients with intracranial hypertension:
- Neurologist
- Neurosurgeon
- Interventional Radiologist
- Intensivist
- Neuro-ophthalmologist
- Emergency clinician
- Deterrence and Patient Education
Preventing intracranial hypertension involves addressing modifiable risk factors and promoting overall brain and eye health. General preventive measures include the following:
- Maintaining a healthy weight to reduce IIH risk
- Regular eye examinations to detect papilledema and other eye abnormalities early
- Managing medications like corticosteroids and oral contraceptives that can give rise to benign intracranial hypertension
- Managing comorbid conditions that may give rise to disorders that cause intracranial hypertension, such as stroke
- Avoiding risky behaviors to prevent TBI
- Genetic counseling for people with a family history of congenital anomalies with associated intracranial hypertension
Patients with IIH should also be educated regarding the condition's potential for disabling blindness. These individuals should consult an ophthalmologist for any visual disturbance.
- Pearls and Other Issues
Intracranial hypertension presents a multifaceted challenge in clinical practice, requiring a nuanced approach to diagnosis and management. Early recognition of symptoms such as severe headaches, visual disturbances, nausea, and vomiting is paramount, prompting a comprehensive evaluation that includes thorough history-taking, physical examination, and diagnostic testing. Once the etiology is identified, treatment strategies for intracranial hypertension should aim to reduce intracranial pressure, alleviate symptoms, and address the underlying cause. Treatment approaches often include a combination of lifestyle modifications, medication management, and, in some cases, surgical interventions. Interprofessional collaboration is essential in navigating the complexities of intracranial hypertension management.
- Enhancing Healthcare Team Outcomes
In the hospital setting, acute intracranial hypertension is best managed by an interprofessional team consisting of a neurologist, neurosurgeon, intensivist, ICU nurses, internist, and pulmonologist. Management is mainly focused on treating and reversing the etiology. Patients often need intensive care and continuous monitoring. Additionally, parameters such as heart rate, blood pressure, body temperature, ventilation, oxygenation, blood glucose, fluid input and output, and electrocardiogram should be monitored. Patients with suspected intracranial hypertension, especially in the context of severe TBI, should also have ICP monitoring.
The primary care clinician counsels patients on IIH's risk factors and weight loss in the outpatient setting. A neurologist should be consulted for headache management. A neuro-ophthalmologist's formal evaluation and monitoring of visual acuity is recommended, as visual function deterioration indicates disease progression and possibly requires surgical intervention.
Collaboration and communication among interprofessional team members are essential for optimizing patient outcomes and providing comprehensive care for individuals with intracranial hypertension. All team members bring unique perspectives and skills to the table, contributing to a comprehensive approach to management that addresses the complex needs of patients with intracranial hypertension from various angles.
- Review Questions
- Access free multiple choice questions on this topic.
- Click here for a simplified version.
- Comment on this article.
Idiopathic Intracranial Hypertension Fundus Examination. Vision loss in the right eye with bilateral disc swelling may indicate idiopathic intracranial hypertension. Fundus photographs a and b reveal bilateral disc edema. The right eye (image b) shows (more...)
Idiopathic Intracranial Hypertension MRI. This is a sagittal T2-weighted MRI brain of a patient with idiopathic intracranial hypertension. Note that an empty sella may be present, though it is not diagnostic of IIH. Contributed by Steve Lange, (more...)
Communicating Hydrocephalus Computed Tomography. Notable features include symmetric ventricular enlargement, periventricular lucency, sulci effacement, and absence of obstructive lesions. Contributed by Monica Gupta, MD
Disclosure: Sandeep Sharma declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies.
Disclosure: Caroline Davidson declares no relevant financial relationships with ineligible companies.
Disclosure: Anil Kumar declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Sharma S, Hashmi MF, Davidson CL, et al. Intracranial Hypertension. [Updated 2024 Mar 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review The Role of Arachnoid Granulations and the Glymphatic System in the Pathophysiology of Idiopathic Intracranial Hypertension. [Curr Neurol Neurosci Rep. 2020] Review The Role of Arachnoid Granulations and the Glymphatic System in the Pathophysiology of Idiopathic Intracranial Hypertension. Mondejar V, Patsalides A. Curr Neurol Neurosci Rep. 2020 May 22; 20(7):20. Epub 2020 May 22.
- Review Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. [Headache. 2013] Review Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Mokri B. Headache. 2013 Jul-Aug; 53(7):1034-53. Epub 2013 Jun 28.
- Venous Sinus Stenting in the Management of Patients with Intracranial Hypertension Manifesting with Skull Base Cerebrospinal Fluid Leaks. [World Neurosurg. 2017] Venous Sinus Stenting in the Management of Patients with Intracranial Hypertension Manifesting with Skull Base Cerebrospinal Fluid Leaks. Iyer RR, Solomon D, Moghekar A, Goodwin CR, Stewart CM, Ishii M, Gailloud P, Gallia GL. World Neurosurg. 2017 Oct; 106:103-112. Epub 2017 Jun 20.
- Review Regulation of brain fluid volumes and pressures: basic principles, intracranial hypertension, ventriculomegaly and hydrocephalus. [Fluids Barriers CNS. 2024] Review Regulation of brain fluid volumes and pressures: basic principles, intracranial hypertension, ventriculomegaly and hydrocephalus. Hladky SB, Barrand MA. Fluids Barriers CNS. 2024 Jul 17; 21(1):57. Epub 2024 Jul 17.
- Invasive and noninvasive means of measuring intracranial pressure: a review. [Physiol Meas. 2017] Invasive and noninvasive means of measuring intracranial pressure: a review. Zhang X, Medow JE, Iskandar BJ, Wang F, Shokoueinejad M, Koueik J, Webster JG. Physiol Meas. 2017 Jul 24; 38(8):R143-R182. Epub 2017 Jul 24.
Recent Activity
- Intracranial Hypertension - StatPearls Intracranial Hypertension - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Idiopathic Intracranial Hypertension: The Monster Within
Aastha takkar.
- Author information
- Article notes
- Copyright and License information
Address for correspondence: Dr. Vivek Lal, Department of Neurology, Post Graduate Institute of Medical Education and Research, Chandigarh - 160012, India. E-mail: [email protected]
Received 2019 Apr 2; Revised 2019 Jun 4; Accepted 2019 Jun 30; Issue date 2020 Mar-Apr.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Idiopathic intracranial hypertension (IIH) is defined as a syndrome of raised intracranial pressure with normal imaging of the brain and cerebrospinal fluid (CSF) composition. There are many controversies and myths that surround IIH. Although patients of IIH may present “typical” symptoms and signs of raised intracranial pressure, clinical scenarios often vary. A typical clinical and radiological finding poses significant problems in diagnosis and management of patients with IIH. We have tried to resolve these controversies and provide a comprehensive update on different aspects of IIH. In this article, we review the common problems encountered while dealing with patients of IIH.
Keywords: Benign intracranial hypertension, idiopathic intracranial hypertension, headache, pseudotumor cerebri syndrome, visual loss
I NTRODUCTION
The term “benign intracranial hypertension” (BIH) was first introduced by Foley.[ 1 ] Several decades later the “not so benign” nature of the entity was recognized by Corbett and Thompson, changing its name from BIH to “idiopathic intracranial hypertension” (IIH)” in 1989.[ 2 ] The diagnostic criteria for IIH were first formulated in 1937 by Dandy and were later modified by Smith in 1985.[ 3 , 4 ] In 2013, Friedman et al . further refined the diagnostic criteria and proposed the condition best described under the umbrella term of pseudotumor cerebri syndrome (PTCS) classifying it into primary or secondary (IIH) depending on the absence or presence of an identifiable cause[ 5 ] [ Table 1a ]. As a result, IIH acts as a subset within the primary PTCS category. The International Headache Society”s International Classification of Headache Disorders 3 rd ed.ition (ICHD-3), 2018 defines IIH under “Headaches attributed to non vascular intracranial disorders”/Headache attributed to increased CSF pressure (ICHD-3, 7.1.1). As per ICHD-3, IIH is described as a new-onset headache or significant worsening of a preexisting headache accompanied by clinical symptoms/signs, and/or neuroimaging signs of raised increased intracranial pressure (ICP) [ Table 1b ].[ 6 ]
Diagnosis of IIH
It is clinically relevant to note that documentation of an elevated CSF pressure (≥250 mm in adults and ≥280 mm in children) is mandatory to establish the diagnosis of “definite” PTCS but the diagnosis of “probable” PTCS may be kept in patients with strongly suggestive clinical history, bilateral papilledema, supportive neuroimaging and “ normal” CSF opening pressure [ Table 1a ]. As CSF pressure may vary in a given individual at varied times of the day, this definition may enable to diagnose such patients of IIH with higher certainty.[ 5 ]
P ATHOPHYSIOLOGY O F IIH: T HE Q UEST B EGINS
Myth: IIH occurs only in obese women
For long it has been believed that IIH occurs exclusively in overweight women of childbearing age group. A meta-analysis and systematic review identified 15 studies and depicted a pooled incidence rate of 1.20/100,000. Various studies have predicted that women are eight times more prone to develop IIH as compared to men.[ 7 , 8 , 9 , 10 ] Digre et al . in their study including 29 male patients of IIH found that clinical characteristics of men with IIH were similar to age-matched female patients.[ 11 ] Similar findings were noted by Kesler et al . in their retrospective review involving 141 IIH patients.[ 12 ]
Bruce et al . studied clinical and radiological characteristics of IIH in 721 consecutive patients (9% males) and noted that males with IIH have different symptom expression and/or different symptom threshold. Males were twice as likely as females to develop severe visual loss, probably because of lack of reporting of nonvisual symptoms like headache, Transient visual obscurtions (TVOS), and tinnitus.[ 13 ]
There is no denying that obesity and IIH are related. Several epidemiological studies have noted that more than 80 to 90% patients of IIH are overweight.[ 10 ] Nonobese patients with a history of a recent weight gain are more inclined to develop IIH.
In a retrospective cohort of 407 consecutive adult patients of IIH, Bruce et al . noted that 84% of patients had a BMI above 30 kg/m 2 ,[ 14 ] while patients with BMI >40 kg/m 2 were noted to have a worse prognosis in another retrospective review by Szweka et al . (2013).[ 15 ] Several weight-loss studies suggest that any amount of weight loss is beneficial in these patients and should be encouraged. Visual field improvement was also noted in the Longitudinal Idiopathic Intracranial Hypertension Treatment Trial (LIIHTT) subjects who never received pharmacological therapy but were only advised lifestyle modification and weight-loss.[ 16 ]
It is important to recognize that though obesity is one of the major contributory factors; it is not the sole factor responsible in the causation of IIH. There is an interplay between various factors and hence patients of either gender with varied BMI may develop IIH.[ 17 ]
D ECEPTIONS A ND E XCEPTIONS: C ONTROVERSIES I N C LINICAL P ROFILE- W HY W E M ISS I T ?
Myth: IIH cannot occur without headache
Headache is one of the most common symptoms with highly variable severity.
Headache due to increased intracranial pressure is often described as throbbing or bursting and is precipitated by factors that increase ICP such as bending, coughing, sneezing, or exertion. Classically, such headache has an early morning worsening (attributed to raised ICP at night as a consequence of recumbent position, raise PCO 2 during sleep due to respiratory depression, and probably decrease CSF absorption).[ 18 ] Early diagnosis of IIH is often based upon the characteristics of headache (e.g. illustrative case). Symptoms such as nausea and vomiting are common and usually occur after waking, thereby frequently accompanying morning headaches.
It is often noted that the pattern of headache changes over time and depends upon the stage of IIH. Headache can be because of increased intracranial pressure, migraine, medication overuse, tension-type headache, low-pressure headache after a lumbar puncture, or iatrogenic Chiari malformation post shunting procedure. Headache was also found to be a significantly disabling symptom and was associated with a poorer quality of life in the neuro-ophthalmology research disease investigator consortium (NORDIC)-IIHTT. About 84% of patients complained of headache in IIHTT cohort, of which 52% described their headaches as migrainous, 22% had tension-type headaches (TTH), 16% had probable migraines and 4% had probable TTH.[ 19 , 20 ]
Freidman et al . in 2002 reviewed medical records of 82 patients with IIH and noted that these patients frequently have a headache due to causes other than IIH.[ 19 ] A small fraction of patients may be even present without any headache.
Other symptoms of IIH
Unilateral or bilateral TVOs occur in around 70% of patients with IIH.[ 8 ] The TVOs are attributed to an increase in CSF pressure around the optic nerve causing disturbances in the microcirculation of optic nerve.[ 21 ]
Around 60% patients may be present with unilateral or bilateral pulsatile or pulse-synchronous tinnitus, which occurs due to the flow turbulence in venous sinuses, due to the heightened transmission of normal vascular pulsations, or due to increased CSF around the cochlear organs.[ 22 ]
Cranial nerve paresis may occur commonly, secondary to increased intracranial pressure. Abducens nerve paresis is commonly manifesting as diplopia in these patients.
Importantly, either of the clinical symptoms may be present (or absent) in any combination. A high index of suspicion in an appropriate clinical setting is necessary to clinch the diagnosis.
Neuro-ophthalmological examination
Myth- IIH cannot occur without papilledema
Papilledema is usually bilateral and is considered as a hallmark sign of IIH [ Figure 2 ]. A validated scale (Modified Frisens Scale) has been used to grade papilledema[ 23 ] [ Table 2 ]. Figure 2 shows representative fundus photographs depicting various stages of papilledema. Rarely, papilledema may be asymmetric, unilateral, or absent. This might be related to complete, partial, or “compartmentalized” obliteration of the subarachnoid space around the optic nerve, which does not allow raised ICP to be transmitted to subarachnoid space around the optic nerve. Further, in patients with recurrent IIH fibrosis of the nerve fiber layer or optic atrophy, it may preclude the development of papilledema underscoring the documentation of raised CSF pressure in such cases.
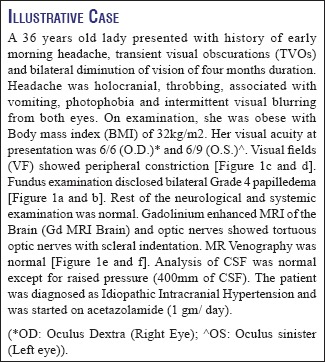
Showing- a: Grade 1 papilledema; b: Grade 2 papilledema, c: Grade 3 papilledema; d: Grade 4 papilledema and e: Grade 5 papilledema
Showing key features from modified Frisens scale for grading disc edema[ 22 ]
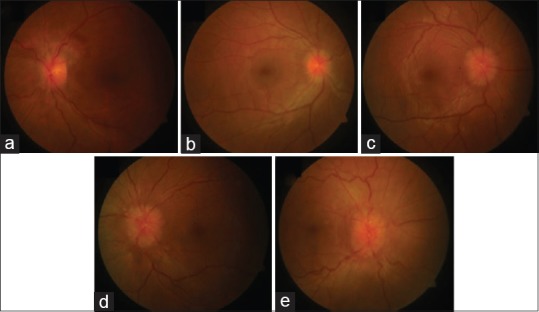
Showing bilateral papilledema (a, b) on fundus examination; Normal visual field of the right eye (c) and constriction of field and enlarged blind spot of the left eye (d). MRI Brain showing Tortuous optic nerves (e) and Normal MR Venography (f)
Digre et al . in their cross-sectional analysis of 353 IIH patients between 1990 and 2003 noted the prevalence of IIH to be 5.7% (20 out of 353 IIH patients without papilledema [IIHWOP]). Patients of IIHWOP were similar in clinical characteristics and presenting visual acuity but mean CSF opening pressure was found to be lower in these patients.[ 24 ]
In another prospective observational study, Favoni et al . reported a prevalence of 2.5% of IIHWOP in patients with chronic refractory headache.[ 25 ]
While MRI brain is generally normal in IIH, raised ICP itself can produce few MRI changes unique to raised ICP which may be helpful in settings of IIHWOP. These include empty sella turcica, flattening of the posterior portion of the globe, distension of optic nerve sheath, tortuosity of the optic nerve sheath, deformity of the pituitary, protrusion and enhancement of optic nerve head, slit-like ventricles, and tight CSF spaces [ Figure 3 ].
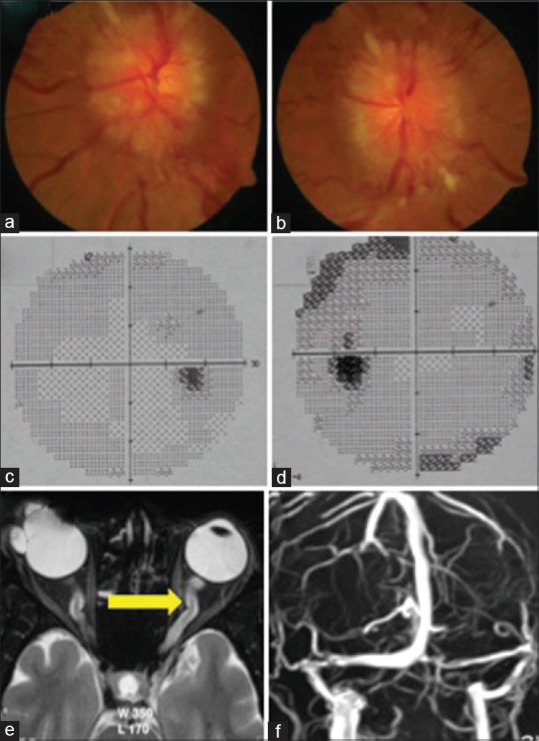
Showing MRI findings in IIH. (a) Showing Distension of Optic Nerve Sheath (T2 Weighted; axial view); (b) Showing Distension of Optic Nerve Sheath (T2 Weighted; coronal view); (c) Showing Tortuosity of Optic Nerve (Blue Arrow) and Scleral Indentation (Green Arrow)
While IIH can occur with or without asymmetrical papilledema, there is a major caveat in making the diagnosis of IIHWOP. Simply having “Headache and elevated pressure” is not enough. In the absence of an appropriate setting, an elevated CSF opening pressure may itself be nonspecific or nondiagnostic.[ 26 , 27 ]
Vision and IIH
Visual dysfunction in IIH is consequent to increased pressure in subarachnoid space around the optic nerve, which results in either or both of the following two processes (Flowchart 1):
Disruption of axonal transport and
Intraneuronal optic nerve ischemia.[ 2 , 28 ]
Visual field (VF) defects are common in patients with IIH. The enlarged blind spot is the commonest followed by loss of infero-nasal portion of the visual field. Gradual depression of peripheral field may ensue in untreated patients with IIH.[ 8 ]
Visual field reading center (VFRC) evaluated 660 baseline VFs from 165 enrolled patients in IIHTT to characterize the VF at the baseline. The most common type of abnormality was a localized nerve fiber bundle-like defect (60%). Localized inferior hemifield loss was more common than superior hemifield loss.[ 29 ]
Visual abnormalities in IIH also occur because of retinal damage. This may be in the form of either (i) a neurosensory detachment or (ii) choroidal folds. In a subgroup analysis of IIHTT, Sibony et al . noted the frequency, patterns, associations, and biomechanical implications of retinal and choroidal folds in papilledema due to IIH. These folds are considered to be the biomechanical signs of stress/strain on the optic nerve head and load-bearing structures induced by intracranial hypertension.[ 30 ]
Visual loss in IIH
Myth: Visual loss cannot occur in IIH
Most often patients of IIH have preserved visual acuity and therefore the major brunt of visual symptoms is on the field of vision (Illustrative case). However, few patients of IIH may also develop significant visual loss which can be transient, acute, or chronic. Transient visual loss in IIH develops secondary to intermittent raised pressure in the subarachnoid sheath surrounding the optic nerve head causing axoplasmic flow stasis and ischemia.
A few patients may develop an acute, severe, and rapidly progressive visual loss. An acute onset of symptoms and signs of intracranial hypertension (less than 4 weeks between onset of initial symptoms and severe visual loss) and rapid worsening of visual loss over a few days in patients of IIH is termed as “Fulminant IIH.”[ 31 ] As noted by Thambisetty et al . in their study including 16 patients, presentation with Fulminant IIH requires urgent surgery.[ 31 ] Acute visual loss often develops due to a sudden increase in intracranial pressure causing a vascular compromise in the optic nerve head causing massive papilledema and peripapillary retinal hemorrhages.
Most commonly, visual loss in IIH develops on a chronic basis in untreated patients of IIH who subsequently develop secondary optic atrophy.
The visual loss at presentation has been associated with worse prognosis by Takkar et al . in their study including 40 IIH patients.[ 9 ]
Thus, the usual course of IIH causes visual dysfunction in the form of slowly spreading disc edema and corresponding constricting visual fields. Awareness about “Fulminant IIH” is necessary to prevent catastrophic visual morbidity associated with it.
Controversies in diagnosis: CSF analysis
Myth- CSF pressure has to be high in IIH at all times
There are several issues concerning the method for measurement of CSF pressure as well as regarding its normal values. Reference level for CSF pressure measurement should be at the level of the left atrium whether done in a supine, prone, or sitting position. ICHD-3 also mentions that CSF pressure should be measured in the absence of ICP lowering treatment in patients of suspected IIH. No sedative must be used before monitoring and the pressure should be measured in lateral decubitus position. CSF pressure may vary during the course of a day and a single measurement may not be indicative of the average CSF pressure over the day. Repeat puncture or a prolonged lumbar/intraventricular pressure monitoring may be required in cases of diagnostic uncertainty.[ 6 , 32 ]
Spuriously high values can occur with Valsalva and with hypoventilation associated with sedation.[ 18 ] Moreover, the reference range for cerebrospinal fluid (CSF) opening pressure in children is higher as compared to their adult counterparts. As defined in the diagnostic criteria for the diagnosis of IIH, opening CSF pressure of more than 280 mm of CSF should be considered abnormal in obese children.[ 6 , 33 ]
Spuriously, low values can occur with hyperventilation (e.g. anxiety) due to reduction in carbon dioxide levels and multiple needle punctures. CSF pressure fluctuates throughout the day and for that simple reason, a single normal CSF measurement does not exclude IIH as the diagnosis.[ 34 ]
As mentioned above, the revised diagnostic criteria also suggest that in an otherwise typical patient, the diagnosis of “probable” IIH may be considered even with normal CSF pressure. The CSF pressure has to be considered in light of the patient”s clinical status.[ 5 ]
Controversies in managing IIH: Medicine or surgery!
The main aim of treatment in IIH is to preserve visual function, to provide symptomatic relief of symptoms (particularly headache) and to treat the potential etiologic factors. The treatment is guided by the “predicted threat to vision” in these patients.[ 35 ] Our protocol of managing patients of IIH is given in Flowchart 2.
C AN IIH B E P REVENTED ?
Role of dietary modification.
A sustained weight loss of approximately 6% from the baseline has been considered to be associated with good visual outcome.[ 36 ] The IIHTT noted an excellent and persistent response even in the placebo arm which only received low sodium, weight reduction diet, and lifestyle management.[ 8 , 37 ] Above all, visual field improvement was also noted in 12% of the Longitudinal IIH Treatment Trial subjects who were never treated with acetazolamide (ACZ) (and had only received lifestyle modification). Effects of gastric weight reduction surgery have been repeatedly evaluated but given the considerable complications, risks should be balanced with benefits before attempting surgery.[ 38 ]
M EDICAL T HERAPY
Acetazolamide: when to use, how much to use, how long to use.
ACZ has been considered as the mainstay of medical management. Its carbonic anhydrase inhibitor effect impedes CSF formation at the choroid plexus. A Cochrane systematic review in 2015 identified two randomized control trials for the use of ACZ in IIH.[ 39 , 40 ]
In patients with any visual loss or refractory symptoms, it is advisable to initially start ACZ at a dose of 500 mg twice a day with a schedule to increase the dose by 250 mg every six days up to a maximum of 4 g daily or a maximally tolerated dose. The IIHTT has provided Class 1 evidence that ACZ is beneficial in patients with IIH with mild visual loss.
Adverse reactions like hypokalemia, allergic reactions, fatigue, paresthesia's, dysgeusia, vomiting, diarrhea, nausea, and fatigue may occur. A few patients may observe metabolic acidosis, kidney stones, transaminitis, pancreatitis, diverticulitis, and renal failure. It is contraindicated in subjects with allergies to sulfa drugs.[ 40 ]
The duration of use of ACZ is also unsettled. In the IIHTT ACZ was tapered off at 6 months in subjects with papilledema of a grade less than one unless they had persistent symptoms or deficits in their visual field. Patients who were continued on ACZ were noted to have continued improvement in the visual functions when followed until 12 months.[ 40 ]
We conclude that the duration of ACZ should be governed by the resolution of clinical symptoms, papilledema, and visual field deficits. Preferably, ACZ should be continued for a period of 6 to 12 months after resolution of papilledema to prevent recurrences.
Are other drugs helpful in IIH?
The carbonic anhydrase inhibitor activity of topiramate makes it an important alternative to ACZ (specifically in patients with contraindication to ACZ). In an open-label study, Celebisoy et al . compared ACZ to topiramate prospectively in 40 patients and concluded that topiramate is as effective as ACZ in treatment of IIH. Weight reduction and reduction of CSF formation were considered as possible mechanisms of action. A role for topiramate in IIH is established at a dose of 25 mg to 200 mg. It's potential to cause weight loss may be an advantage to obese patients. Caution is advised in patients with sulfa allergies, glaucoma, and nephrolithiasis. Other side effects include paresthesia's, depression, cognitive slowing, weight loss, and potential teratogenic risks. It may reduce the efficacy of oral contraceptive drugs.[ 41 ]
The role of other diuretics such as furosemide and amiloride is not certain hence combining diuretics may lead to severe hypokalemia and therefore is best avoided.
How to treat headache in IIH?
The type of headache should guide its treatment and an early introduction of prophylactic therapy should be considered. The patients may have varied types of headache and an adequate caution needs to be observed while selecting the drugs (e.g. propensity of weight-gain by sodium valproate, tricyclic antidepressants, beta -blockers, etc.).[ 19 , 35 ]

What is the role of steroids in IIH?
While the use of long-term steroids have been noted to cause/precipitate IIH, high-dose pulse steroids may be used as a temporary measure while awaiting definitive surgical procedure in patients presenting acute, severe visual loss.[ 42 ] Thambisetty et al . also considered the use of steroids in four of their 16 patients with fulminant IIH.[ 31 ]
Whence surgical measures?
The patients, who develop rapid progression of visual loss, are medically refractory or who present an acute severe visual loss require urgent CSF diversion. As already mentioned, temporary measures like high-dose pulse steroids; repeated lumbar puncture or lumbar drainage may be attempted while awaiting these definitive measures.
The controversy still remains as to which surgical procedure should be preferred.[ 43 ] The various surgical options available are (a) optic nerve sheath fenestration (ONSF) or (b) CSF diversion procedures- ventriculoperitoneal shunt (VP Shunt)/Ttheco-peritoneal shunt (TP Shunt). ONSF has been preferred when visual symptoms are predominant or the visual compromise is unilateral. Authors preferring ONSF to CSF diversion techniques have advocated low morbidity, infection rates, and mortality. Local complications may occur e.g. retrobulbar hemorrhage, orbital hematoma, orbital apex syndrome, orbital cellulitis, traumatic optic neuropathy, heterotopias, diplopia, peripapillary hemorrhages, disc hemorrhage, cyst formation, and conjunctival abscess. Formation of synechia, pupillary abnormalities, and late failure may also occur.
Authors advocating CSF diversion procedures argue that these procedures are more beneficial in patients presenting with prominent nonvisual dysfunction while providing benefit in regaining visual functions as well. A direct threat to vision may occur in patients undergoing ONSF, and in intractable cases, a CSF diversion procedure may be required after ONSF. Risk of shunt-related complications like an infection; shunt migration or obstruction, subdural hemorrhages, over drainage and tonsillar herniation need to be borne in mind while proceeding for CSF diversion.
There is a need for studies, which directly compare ONSF with CSF-shunting procedures for the treatment of IIH. As of now, the decision rests on the local preference, prompt availability and access to the procedure and surgical expertise.[ 43 ]
What is the current role of neurovascular stenting in acute IIH to prevent loss of vision?
Transverse sinus stenting in IIH is equally controversial. Starke et al . identified 17 studies including 185 patients who underwent 221 stenting procedures. A systematic review of these patients suggested that neurovascular stenting could be a safe and effective therapeutic option for medically refractory IIH.[ 44 ] Cappuzzo et al . in 2018 also concluded that transverse sinus stenosis is an effective therapeutic option in patients with IIH.[ 45 ] While recent literature claims that neurovascular stenting is a safe alternative, the available literature is based upon small or non-randomized case series and their reviews.[ 45 ] To conclude, evidence on neurovascular stenting is limited and long-term follow-up data is not available.[ 35 ]
How to follow patients of IIH?
In a recently published consensus guideline on management of IIH reflecting practices from across UK, importance of documentation and serial follow up of visual acuity, pupil examination, formal visual field assessment, dilated fundus examination, and grading of papilledema and BMI calculation being stressed.[ 35 ] In general, we believe that the patient presenting with progressive or severe visual loss should be followed every two weeks until the visual functions stabilize. Patients with moderate visual loss and stable visual functions also need to be followed every 1 to 3 months depending upon the grade of papilledema and clinical signs and symptoms. Patients with mild disc edema may also be followed every 3 to 6 months if the visual functions and clinical symptoms have stabilized.
C ONTROVERSIES I N I IH W ITH P REGNANCY
Why iih in pregnancy.
At several instances, IIH may first appear during pregnancy in otherwise healthy women. Sudden weight gain and hormonal fluctuations during pregnancy may be causative factors although the last word is yet to be said.
How to treat IIH in pregnancy?
For patients with minimal/stable clinical and visual functions, only follow-up is required once the diagnosis is established and secondary causes have been ruled out. The disorder may itself resolve or remit after delivery. However, in patients with mild-to-moderate visual loss or deteriorating clinical and visual symptoms, definitive treatment may be required. It is believed that the management of pregnant patients with IIH should be the same as non pregnant patients except calorie restriction or the use of diuretics. ACZ is labeled as a category C medication in pregnancy given its teratogenic potential. Use of ACZ after 20 weeks of pregnancy is often considered safe but a clear risk-benefit ratio should be considered before advising it.[ 46 ] The visual outcome is considered to be similar to non pregnant women. Topiramate should not be advised during pregnancy.[ 47 ] A clear risk-benefit assessment should be advocated while choosing agents for headache management during pregnancy. Serial lumbar punctures or ONSF may be considered in patients with imminent risks of visual loss.[ 48 ]
Does IIH have an implication on the mode of delivery/anesthesia?
No specific mode of delivery is suggested for patients with IIH. The mode of delivery should be largely governed by obstetric factors. The attempt may be made to avoid prolonged Valsalva during the second stage of labor if deemed feasible in appropriate settings. Adequate analgesia should be administered during labor. Spinal anesthesia or epidural anesthesia has been found to be safe. In operated patients of IIH with preexisting LP shunt, general anesthesia for cesarean section has been recommended over epidural anesthesia.[ 49 ]
IIH in children
None of the age group is immune to IIH. However, there is an increased incidence noted in the adolescent age group as compared to the younger population. No correlation with obesity or female gender is noted. Excluding secondary structural, endocrinal, nutritional and metabolic causes, it becomes important while dealing with this age group. The management protocol is largely the same as followed in the adult counter parts. Outcomes are usually encouraging though in rare cases devastating visual loss may occur.[ 5 , 50 ]
C ONCLUSIONS
IIH is probably the most common “neurological” cause of preventable visual morbidity. It is important to identify subgroups of patients with “eye at risk” to salvage vision. Awareness of typical presentations and prompt recognition are of paramount importance to prevent complications.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
R EFERENCES
- 1. Foley J. Benign forms of intracranial hypertension; toxic and otitic hydrocephalus. Brain J Neurol. 1955;78:1–41. doi: 10.1093/brain/78.1.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–74. doi: 10.1001/archneur.1982.00510200003001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Dandy WE. Intracranial pressure without brain tumor. Ann Surg. 1937;106:492–513. doi: 10.1097/00000658-193710000-00002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5:55–6. [ PubMed ] [ Google Scholar ]
- 5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–65. doi: 10.1212/WNL.0b013e3182a55f17. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. 3rd ed. vol 38. Cephalalgia: 2018. pp. 1–211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45:875–7. doi: 10.1001/archneur.1988.00520320065016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain J Neurol. 1991;114:155–80. [ PubMed ] [ Google Scholar ]
- 9. Takkar A, Goyal MK, Bansal R, Lal V. Clinical and neuro-ophthalmologic predictors of visual outcome in idiopathic intracranial hypertension. Neuro-Ophthalmol Aeolus Press. 2018;42:201–8. doi: 10.1080/01658107.2017.1400570. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. McCluskey G, Doherty-Allan R, McCarron P, Loftus AM, McCarron LV, Mulholland D, et al. Meta-analysis and systematic review of population-based epidemiological studies in idiopathic intracranial hypertension. Eur J Neurol. 2018;25:1218–27. doi: 10.1111/ene.13739. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol. 1988;45:866–72. doi: 10.1001/archneur.1988.00520320056015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Kesler A, Goldhammer Y, Gadoth N. Do men with pseudomotor cerebri share the same characteristics as women? A retrospective review of 141 cases. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2001;21:15–7. doi: 10.1097/00041327-200103000-00004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–9. doi: 10.1212/01.wnl.0000333254.84120.f5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Bruce BB, Kedar S, Van Stavern GP, Corbett JJ, Newman NJ, Biousse V. Atypical idiopathic intracranial hypertension. Neurology. 2010;74:1827–32. doi: 10.1212/WNL.0b013e3181e0f838. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: Relation between obesity and visual outcomes. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2013;33:4–8. doi: 10.1097/WNO.0b013e31823f852d. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Wall M, Kupersmith MJ, Thurtell MJ, Moss HE, Moss EA, Auinger P, et al. The longitudinal idiopathic intracranial hypertension trial: Outcomes from months 6-12. Am J Ophthalmol. 2017;176:102–7. doi: 10.1016/j.ajo.2017.01.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Chen J, Wall M. Epidemiology and risk factors for idiopathic intracranial hypertension. Int Ophthalmol Clin. 2014;54:1–11. doi: 10.1097/IIO.0b013e3182aabf11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i23–7. doi: 10.1136/jnnp.73.suppl_1.i23. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Friedman DI, Quiros PA, Subramanian PS, Mejico LJ, Gao S, McDermott M, et al. Headache in idiopathic intracranial hypertension: Findings from the idiopathic intracranial hypertension treatment trial. Headache. 2017;57:1195–205. doi: 10.1111/head.13153. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Sina F, Razmeh S, Habibzadeh N, Zavari A, Nabovvati M. Migraine headache in patients with idiopathic intracranial hypertension. Neurol Int. 2017;9 doi: 10.4081/or.2017.7280. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5641834/ [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Sadun AA, Currie JN, Lessell S. Transient visual obscurations with elevated optic discs. Ann Neurol. 1984;16:489–94. doi: 10.1002/ana.410160410. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G, et al. Idiopathic intracranial hypertension: The prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–24. doi: 10.1212/01.wnl.0000066683.34093.e2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Frisén L. Swelling of the optic nerve head: A staging scheme. J Neurol Neurosurg Psychiatry. 1982;45:13–8. doi: 10.1136/jnnp.45.1.13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Digre KB, Nakamoto BK, Warner JEA, Langeberg WJ, Baggaley SK, Katz BJ. A Comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49:185–93. doi: 10.1111/j.1526-4610.2008.01324.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Favoni V, Pierangeli G, Toni F, Cirillo L, La Morgia C, Abu-Rumeileh S, et al. Idiopathic Intracranial Hypertension Without Papilledema (IIHWOP) in chronic refractory headache. Front Neurol. 2018;9 doi: 10.3389/fneur.2018.00503. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6029151/ [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Digre KB. Not so benign intracranial hypertension. BMJ. 2003;326:613–4. doi: 10.1136/bmj.326.7390.613. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Digre KB. Three current controversies in idiopathic intracranial hypertension. Neuroophthal. 2009;33:93–9. [ Google Scholar ]
- 28. Hung HL, Kao LY, Huang C-C. Ophthalmic features of idiopathic intracranial hypertension. Eye Lond Engl. 2003;17:793–5. doi: 10.1038/sj.eye.6700443. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Keltner JL, Johnson CA, Cello KE, Wall M. NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) Invest Ophthalmol Vis Sci. 2014;55:3200–7. doi: 10.1167/iovs.14-14243. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Sibony PA, Kupersmith MJ, Feldon SE, Wang JK, Garvin M. Retinal and choroidal folds in papilledema. Invest Ophthalmol Vis Sci. 2015;56:5670–80. doi: 10.1167/iovs.15-17459. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68:229–32. doi: 10.1212/01.wnl.0000251312.19452.ec. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Olesen J. International classification of headache disorders. Lancet Neurol. 2018;17:396–7. doi: 10.1016/S1474-4422(18)30085-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Avery RA, Licht DJ, Shah SS, Huh JW, Seiden JA, Boswinkel J, et al. CSF opening pressure in children with optic nerve head edema. Neurology. 2011;76:1658–61. doi: 10.1212/WNL.0b013e318219fb80. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–21. doi: 10.1136/jnnp.2003.033126. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, et al. Idiopathic intracranial hypertension: Consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89:1088–100. doi: 10.1136/jnnp-2017-317440. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50:1094–8. doi: 10.1212/wnl.50.4.1094. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Smith SV, Friedman DI. The idiopathic intracranial hypertension treatment trial: A review of the outcomes. Headache. 2017;57:1303–10. doi: 10.1111/head.13144. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Handley JD, Baruah BP, Williams DM, Horner M, Barry J, Stephens JW. Bariatric surgery as a treatment for idiopathic intracranial hypertension: A systematic review. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2015;11:1396–403. doi: 10.1016/j.soard.2015.08.497. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Piper RJ, Kalyvas AV, Young AMH, Hughes MA, Jamjoom AAB, Fouyas IP. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2015:CD003434. doi: 10.1002/14651858.CD003434.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: The idiopathic intracranial hypertension treatment trial. JAMA. 2014;311:1641–51. doi: 10.1001/jama.2014.3312. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Celebisoy N, Gökçay F, Sirin H, Akyürekli O. Treatment of idiopathic intracranial hypertension: Topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007;116:322–7. doi: 10.1111/j.1600-0404.2007.00905.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Liu GT, Glaser JS, Schatz NJ. High-dose methylprednisolone and acetazolamide for visual loss in pseudotumor cerebri. Am J Ophthalmol. 1994;118:88–96. doi: 10.1016/s0002-9394(14)72847-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Spitze A, Malik A, Al-Zubidi N, Golnik K, Lee AG. Optic nerve sheath fenestration vs cerebrospinal diversion procedures: What is the preferred surgical procedure for the treatment of idiopathic intracranial hypertension failing maximum medical therapy? J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2013;33:183–8. doi: 10.1097/WNO.0b013e318292d06f. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Starke RM, Wang T, Ding D, Durst CR, Crowley RW, Chalouhi N, et al. Endovascular treatment of venous sinus stenosis in idiopathic intracranial hypertension: Complications, neurological outcomes, and radiographic results. Sci World J. 2015;2015:140408. doi: 10.1155/2015/140408. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Cappuzzo JM, Hess RM, Morrison JF, Davies JM, Snyder KV, Levy EI, et al. Transverse venous stenting for the treatment of idiopathic intracranial hypertension, or pseudotumor cerebri. Neurosurg Focus. 2018;45:E11. doi: 10.3171/2018.5.FOCUS18102. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Falardeau J, Lobb BM, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2013;33:9–12. doi: 10.1097/WNO.0b013e3182594001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Weston J, Bromley R, Jackson CF, Adab N, Clayton-Smith J, Greenhalgh J, et al. Monotherapy treatment of epilepsy in pregnancy: Congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11:CD010224. doi: 10.1002/14651858.CD010224.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Tang RA. Management of idiopathic intracranial hypertension in pregnancy. Medscape Gen Med. 2005;7:40. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Tang RA, Dorotheo EU, Schiffman JS, Bahrani HM. Medical and surgical management of idiopathic intracranial hypertension in pregnancy. Curr Neurol Neurosci Rep. 2004;4:398–409. doi: 10.1007/s11910-004-0087-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Ko MW, Liu GT. Pediatric idiopathic intracranial hypertension (Pseudotumor Cerebri) Horm Res Paediatr. 2010;74:381–9. doi: 10.1159/000321180. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (892.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
When viewing this topic in a different language, you may notice some differences in the way the content is structured, but it still reflects the latest evidence-based guidance.
Idiopathic intracranial hypertension
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Idiopathic intracranial hypertension (IIH) is a disorder of increased intracranial pressure in an alert and orientated patient. The most popular hypothesis is that IIH is a syndrome of reduced cerebrospinal fluid absorption.
Clinical features include headaches, pulse-synchronous tinnitus, transient visual obscurations, visual loss, neck and back pain, and diplopia.
Signs include papilloedema, sixth nerve paresis, and disturbances in sensory visual function. Visual field loss is ubiquitous, and the prototype pattern for early loss is enlargement of the blind spot and inferonasal loss. Diagnostic criteria are the modified Dandy criteria.
In all patients with IIH, treatment consists of eliminating causal factors, such as drugs and other conditions known to cause increased intracranial pressure, and instituting a low-sodium weight-reduction diet plus acetazolamide when indicated. Therapy can be given to reverse and prevent loss of vision.
IIH, also known as pseudotumor cerebri, is a disorder of increased intracranial pressure that occurs mainly in overweight women of childbearing years, often in the setting of weight gain. [1] Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010 Aug;28(3):593-617. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908600 http://www.ncbi.nlm.nih.gov/pubmed/20637991?tool=bestpractice.com Its cause is unknown. It is characterised by increased intracranial pressure and its associated signs and symptoms in an alert and orientated patient but without localising neurological findings. There is no evidence of deformity or obstruction of the ventricular system, and neurodiagnostic studies are normal except for increased cerebrospinal fluid pressure and the related neuroimaging signs. Furthermore, no secondary cause of intracranial hypertension is apparent. IIH can either be self-limited or have a lifelong chronic course. [1] Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010 Aug;28(3):593-617. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908600 http://www.ncbi.nlm.nih.gov/pubmed/20637991?tool=bestpractice.com [2] Shah VA, Kardon RH, Lee AG, et al. Long-term follow-up of idiopathic intracranial hypertension: the Iowa experience. Neurology. 2008 Feb 19;70(8):634-40. http://www.ncbi.nlm.nih.gov/pubmed/18285538?tool=bestpractice.com
History and exam
Key diagnostic factors.
- presence of risk factors
- visual field loss
Other diagnostic factors
- transient visual obscurations
- pulse-synchronous tinnitus
- photophobia
- retrobulbar pain
- optical disc swelling
- decreased visual acuity
- ocular motility disturbances
- relative afferent pupillary defect
Risk factors
- obesity and weight gain
- certain medication use
- associated causal diseases
- sleep apnoea
- family history
Diagnostic investigations
1st investigations to order.
- visual field testing (perimetry)
- dilated fundoscopy
- visual acuity
- MRI of brain with or without contrast
- lumbar puncture at spinal L3/L4
Investigations to consider
- magnetic resonance venogram of head
- optical coherence tomography
Treatment algorithm
All patients, contributors, michael wall, md.
Department of Neurology and Department of Ophthalmology & Visual Sciences
University of Iowa Hospitals & Clinics and Iowa City VA Health Care System
Disclosures
MW is an author of a number of references cited in this topic.
Mansoor Mughal, MD
Retina Fellow
Rutgers University
Robert Wood Johnson University Hospital
New Brunswick
MM declares that he has no competing interests.
Peer reviewers
Paul w. brazis, md.
Consultant in Neurology and Neuro-Ophthalmology
Mayo Clinic Florida
Jacksonville
PWB declares that he has no competing interests.
Tim D. Matthews, MBBS
Consultant Neuro-ophthalmologist
Birmingham Neuro-ophthalmology Unit
University Hospital Birmingham
TDM declares that he has no competing interests.

Differentials
- Intracranial structural anomalies
- European Headache Federation guideline on idiopathic intracranial hypertension
Patient information
Obesity - drugs and surgery
Obesity - diet and exercise
Use of this content is subject to our disclaimer
Log in or subscribe to access all of BMJ Best Practice
Log in to access all of bmj best practice, help us improve bmj best practice.
Please complete all fields.
I have some feedback on:
We will respond to all feedback.
For any urgent enquiries please contact our customer services team who are ready to help with any problems.
Phone: +44 (0) 207 111 1105
Email: [email protected]
Your feedback has been submitted successfully.
Idiopathic Intracranial Hypertension
(benign intracranial hypertension; intracranial venous hypertension; pseudotumor cerebri).
Idiopathic intracranial hypertension causes increased intracranial pressure without a mass lesion or hydrocephalus, probably by obstructing venous drainage; cerebrospinal fluid composition is normal.
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Key Points |
(See also Approach to the Patient With Headache .)
Idiopathic intracranial hypertension typically occurs in women of childbearing age. Incidence is 1/100,000 in normal-weight women but 20/100,000 in women with obesity. Intracranial pressure (ICP) is elevated (> 250 mm H2O); the cause is unknown but can involve obstruction of cerebral venous outflow, which can result from increased CSF pressure which can result in increased venous obstruction—a vicious circle.
Symptoms and Signs of Idiopathic Intracranial Hypertension
Almost all patients have a daily or near daily generalized headache of fluctuating intensity, at times with nausea. They may also have transient obscuration of vision, diplopia (due to 6th cranial nerve dysfunction), and pulsatile intracranial tinnitus. Vision loss begins peripherally and may not be noticed by patients until late in the course. Permanent vision loss is the most serious consequence. Once vision is lost, it usually does not return, even if ICP is reduced.
Bilateral papilledema is common; a few patients have unilateral or no papilledema. In some asymptomatic patients, papilledema is discovered during routine ophthalmoscopic examination. Neurologic examination may detect partial 6th cranial nerve palsy but is otherwise unremarkable.
Pearls & Pitfalls
Diagnosis of idiopathic intracranial hypertension.
MRI with magnetic resonance venography
Lumbar puncture
If clinical findings suggest idiopathic intracranial hypertension, clinicians should check visual fields and optic fundi, even in patients with no visual symptoms.
Diagnosis of idiopathic intracranial hypertension is suspected clinically and established by brain imaging (preferably MRI with magnetic resonance venography) that has normal results (except for narrowing of the venous transverse sinus). If not contraindicated, lumbar puncture with cerebrospinal fluid (CSF) testing is then done. Elevated opening pressure and normal CSF composition suggests idiopathic intracranial hypertension.
Use of certain medications and certain disorders can produce a clinical picture resembling idiopathic intracranial hypertension and should be excluded (see table Conditions Associated With Papilledema and Resembling Idiopathic Intracranial Hypertension ).
Conditions Associated With Papilledema and Resembling Idiopathic Intracranial Hypertension
Treatment of idiopathic intracranial hypertension.
Weight loss if needed
Sometimes surgery
Idiopathic intracranial hypertension occasionally resolves without treatment.
Treatment of idiopathic intracranial hypertension is aimed at the following:
Reducing pressure
Preserving vision
Relieving symptoms
Preventive medications used for migraine may relieve headache. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used as needed.
Patients with obesity are encouraged to lose weight, which may help reduce intracranial pressure.
Serial lumbar punctures are controversial but are sometimes used, particularly if, while waiting for definitive treatment, vision is threatened. Definitive treatment includes optic nerve sheath fenestration, shunting, and venous sinus stenting.
Any potential causes (disorders, drugs, or medications) are corrected or eliminated if possible.
Frequent ophthalmologic assessment (including quantitative visual fields) is required to monitor response to treatment; testing visual acuity is not sensitive enough to warn of impending vision loss.
If vision deteriorates despite treatment, one of the following may be indicated:
Optic nerve sheath fenestration
Shunting (lumboperitoneal or ventriculoperitoneal)
Endovascular venous stenting
Bariatric surgery with sustained weight loss may cure the disorder in patients who have obesity and were otherwise unable to lose weight.
Consider idiopathic intracranial hypertension if patients, particularly women with excess body weight, have a daily generalized headache with or without visual symptoms; check visual fields and optic fundi.
Diagnose based on results of brain imaging (preferably MRI with venography) and, if not contraindicated, lumbar puncture; consider chronic meningitis.
Do frequent ophthalmologic assessments (including quantitative visual fields) to monitor response to treatment.
If vision deteriorates despite treatment, consider optic nerve sheath fenestration, shunting, or endovascular venous stenting.

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences


IMAGES
COMMENTS
Jan 2, 2024 · Clinical features History. Symptoms of IIH are usually chronic and progressive and include: 2,4-6. Headache: the most common clinical presentation of IIH.Usually non-specific and diffuse, and sometimes associated with nausea/ vomiting and retrobulbar pain.
Sep 29, 2022 · Pseudotumor cerebri (PTC), also known by the name idiopathic intracranial hypertension, is a disorder with increased intracranial pressure (ICP) and associated headaches, papilledema, vision changes, or pulsatile tinnitus in the setting of normal imaging and cerebrospinal fluid (CSF) studies. It mainly affects overweight women of child-bearing age [1]; however, women of all ages, men, and ...
Nov 4, 2024 · The original terminology was for pseudotumor cerebri syndrome but the term idiopathic intracranial hypertension has since supplanted it. The criteria place patients into one of four diagnostic subgroups: definite idiopathic intracranial hypertension: opening pressure ≥25 cm CSF (H 2 O) and papilledema
Mar 3, 2024 · Intracranial hypertension is a condition characterized by elevated pressure within the skull. The increase in pressure can exert significant stress on the brain and other intracranial structures, potentially leading to a range of neurological symptoms and complications. Intracranial hypertension's clinical manifestations vary depending on the underlying cause, severity of pressure elevation ...
Idiopathic intracranial hypertension is a condition of raised intracranial pressure of unknown cause. Features include new onset headache, which is frequently non-specific; papilloedema is present, visual disturbances are common; and there may be ...
An acute onset of symptoms and signs of intracranial hypertension (less than 4 weeks between onset of initial symptoms and severe visual loss) and rapid worsening of visual loss over a few days in patients of IIH is termed as “Fulminant IIH.” As noted by Thambisetty et al. in their study including 16 patients, presentation with Fulminant ...
Idiopathic intracranial hypertension (IIH), previously known as pseudotumor cerebri and benign intracranial hypertension, is a condition characterized by increased intracranial pressure (pressure around the brain) without a detectable cause. [2] The main symptoms are headache, vision problems, ringing in the ears, and shoulder pain.
Jul 20, 2022 · Idiopathic intracranial hypertension (IIH) is a disorder of unknown etiology that predominantly affects obese women of childbearing age. The primary problem is chronically elevated intracranial pressure (ICP), and the most important neurologic manifestation is papilledema, which may lead to progressive optic atrophy and blindness.
Feb 4, 2022 · Idiopathic intracranial hypertension (IIH) is a disorder of increased intracranial pressure in an alert and orientated patient. The most popular hypothesis is that IIH is a syndrome of reduced cerebrospinal fluid absorption. Clinical features include headaches, pulse-synchronous tinnitus, transie...
Idiopathic intracranial hypertension causes increased intracranial pressure without a mass lesion or hydrocephalus, probably by obstructing venous drainage; cerebrospinal fluid composition is normal. (See also Approach to the Patient With Headache.) Idiopathic intracranial hypertension typically occurs in women of childbearing age.