Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Drug delivery articles from across Nature Portfolio
Drug delivery describes the method and approach to delivering drugs or pharmaceuticals and other xenobiotics to their site of action within an organism, with the goal of achieving a therapeutic outcome. Issues of pharmacodynamics and pharmacokinetics are important considerations for drug delivery.
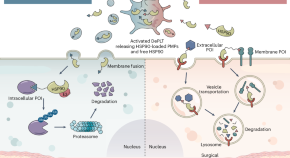
Proteolytic platelets as targeted protein degraders
Proteolytic chimeras constructed from various bioactive modules can degrade either cytoplasmic or extracellular proteins, but their pharmacology faces challenges. Now, a protein degradation platform built with engineered platelets enables the targeted depletion of intracellular and extracellular proteins at hemorrhagic sites, addressing several limitations associated with proteolytic chimeras.
Latest Research and Reviews
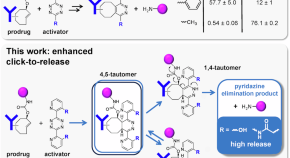
Ortho-functionalized pyridinyl-tetrazines break the inverse correlation between click reactivity and cleavage yields in click-to-release chemistry
The bioorthogonal tetrazine-triggered cleavage of trans -cyclooctene-linked payloads has strong potential in click-to-release drug delivery, however, an inverse correlation between click reactivity and payload release yield is hampering their clinical translation. Here, the authors develop ortho-substituted bis-pyridinyl-tetrazines with hydrogen-bonding hydroxyl or amido groups for efficient tautomerization and payload elimination, achieving release yields of 96% with 18-fold more reactive tetrazines.
- Ron M. Versteegen
- Raffaella Rossin
- Marc S. Robillard
Ginseng glucosyl oleanolate inhibit cervical cancer cell proliferation and angiogenesis via PI3K/AKT/HIF-1α pathway
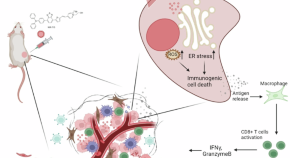
NIR-715 photodynamic therapy induces immunogenic cancer cell death by enhancing the endoplasmic reticulum stress response
- Zhen-Yuan Zheng
Emerging therapeutic strategies to mitigate female and male reproductive aging
People now choose to start families in their 30s to 40s, when fertility is low owing to reproductive aging. In this Perspective, the authors highlight the absence of clinically confirmed therapeutics to mitigate reproductive aging or extend fertility, and they summarize the range of emerging strategies and point to knowledge gaps and future directions in the field.
- Yasmyn E. Winstanley
- Jennifer S. Stables
- Rebecca L. Robker
Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia
A platform for mRNA lipid nanoparticle delivery to the placenta to treat pre-eclampsia is shown to improve fetal and maternal health in mice and has potential clinical applications in obstetric disorders and women’s health.
- Kelsey L. Swingle
- Alex G. Hamilton
- Michael J. Mitchell

The assessment and aetiology of drug-induced ischaemic priapism
- Divyen Moodley
- Anja Badenhorst
News and Comment
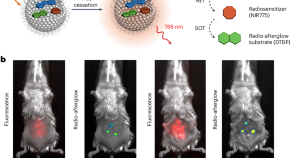
Organic radio-afterglow nanoprobes for cancer theranostics
Leveraging X-rays to induce prolonged luminescence (radio-afterglow) and radiodynamic effects from typically inorganic optical agents enables diagnosis and therapy at light-inaccessible tissue depths. Now, a cascade X-ray energy conversion approach is developed to increase the intrinsically low X-ray conversion efficiency of organic molecules for the construction of radio-afterglow nanoprobes for cancer theranostics.
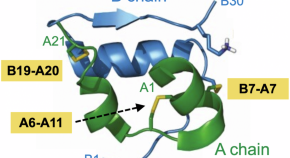
Splitting the chains: ultra-basal insulin analog uncovers a redox mechanism of hormone clearance
Reporting in Nature Communications , Kjeldsen and colleagues describe a redox mechanism of insulin clearance based on separation of A- and B chains. Exploiting an ultra-long-acting analog protected from classical clearance pathways, the study highlights principles of protein stability in pharmacology.
- Michael A. Weiss

Extracellular vesicle gatekeepers for tumours
Cancer-cell-derived extracellular vesicles bind to therapeutics and shuttle them out of the tumour to the liver for destruction.
- Joy Wolfram
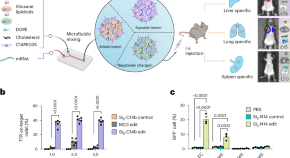
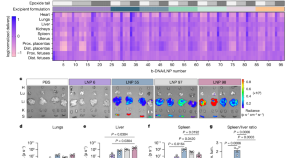
Small structural changes in siloxane-based lipidoids improve tissue-specific mRNA delivery
From a single library of siloxane-based lipidoids, siloxane-incorporated lipid nanoparticles (SiLNPs) involving minor alterations in lipid chemistry yield tissue-specific mRNA delivery to the liver, lung, or spleen. Upon enhanced intracellular delivery, these SiLNPs show clinical promise for protein replacement therapies, regenerative medicine, and CRISPR–Cas-based gene editing applications.
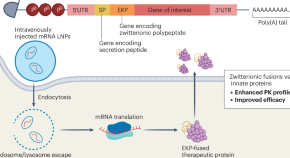
A zwitterionic twist
An mRNA sequence that encodes a zwitterionic polypeptide fused to a therapeutic protein improves the pharmacokinetic properties of mRNA therapeutics.
- Sihan Xiong
- Khalid Shah
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Recent trends and advances in microbe-based drug delivery systems
Pravin shende, vasavi basarkar.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2018 Dec 21; Accepted 2019 Jul 22; Collection date 2019 Dec.
Since more than a decade, pharmaceutical researchers endeavor to develop an effective, safe and target-specific drug delivery system to potentiate the therapeutic actions and reduce the side effects. The conventional drug delivery systems (DDSs) show the improvement in the lifestyle of the patients suffering from non-communicable diseases, autoimmune diseases but sometimes, drug resistance developed during the treatment is a major concern for clinicians to find an alternative and more advanced transport systems. Advancements in drug delivery facilitate the development of active carrier for targeted action with improved pharmacokinetic behavior. This review article focuses on microbe-based drug delivery systems to provide safe, non-toxic, site-specific targeted action with lesser side effects. Pharmaceutical researchers play a vital part in microbe-based drug delivery systems as a therapeutic agent and carrier. The properties of microorganisms like self-propulsion, in-situ production of therapeutics, penetration into the tumor cells, increase in immunity, etc. are of interest for development of highly effective delivery carrier. Lactococcus lactis is therapeutically helpful in Inflammatory Bowel Disease (IBD) and is under investigation of phase I clinical trial. Moreover, bacteria, anti-cancer oncolytic viruses, viral vectors (gene therapy) and viral immunotherapy are the attractive areas of biotechnological research. Virus acts as a distinctive candidate for imaging of tumor and accumulation of active in tumor.
Graphical abstract.
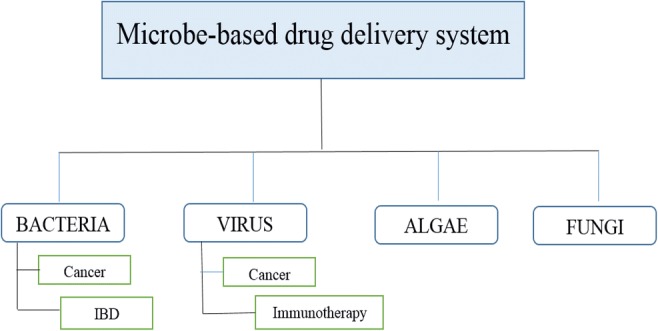
Classification of microbe-based drug delivery system
Keywords: Bacteria, Tumoricidal, Attenuated, Virosomes, Biomolecules
Introduction
Around 70% of global deaths are caused due to non-communicable diseases like cancer, cardiovascular diseases (CVD), diabetes, chronic lungs diseases, etc. The conventional drug therapy (tablets, capsules and pills). uses to treat or manage such life-threatening and infectious diseases but has shown limitations due to cytotoxicity, microbial resistance and adverse drug reaction. To overcome such undesirable effects, scientists have been worked on effective and alternative systems viz. novel drug delivery system, microbe-based delivery systems and gene delivery systems. Ancient literature reported that the microorganisms such as bacteria, virus and fungi-based delivery systems were employed to treat various conditions like cancer, cardiovascular disorder, neurological disorder, etc. It also revealed that microbes are not always pathogenic but because of biological behavior, they reduce noxious and associated harmful effects. [ 1 ]
Microbes especially whole bacteria, bacterial toxin, bacteria ghost or viruses or fungi show some rationale results in delivery system. For example, bacterium like C. novyi NT shows the ability to penetrate and inhibit the growth of a tumor. Besides whole microorganisms, their contents also showed an immuno-stimulant influence. [ 1 ] The virus carrier envelopes potentially deliver drugs, biomolecules like peptides, nucleic acids and genetic material. Moreover, the rebuilt viral envelopes may be articulated carriers for macromolecules like nucleic acids, genes or drugs, known as “Virosomes” [ 2 ]. The cell wall constituents of fungi known as chitin is a latent carrier of many actives, nucleic acids, etc. This review article provides an insight on recent advances of different types of microbe-based delivery systems for treating various diseases like cancer, inflammatory bowel diseases [ 3 ]. Bacillus is another agent used to deliver the drugs and stable throughout the gastric environment. The characteristic bacilli spores were incorporated with curcumin and release of drug was determined the disintegration of outermost coat in alkaline colon medium. These bacilli spores showed promising results for delivering of drug in colon cancer as compared to conventional drug. [ 4 ]
Classification of microorganisms
The microorganisms are classified on the basis of their cell structure as shown in Fig. 1 whereas biohybrid-based drug delivery system is shown in Fig. 2 .
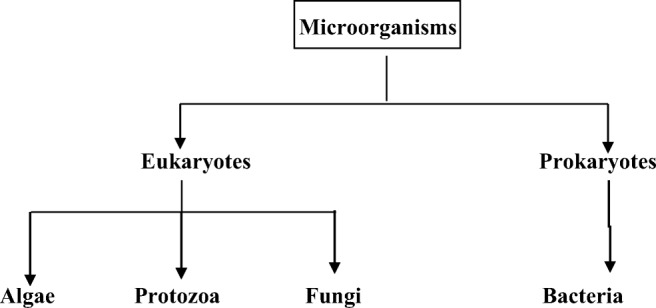
Classification of microorganisms according to cell structure
Biohybrid-based drug delivery system
History of the microbe as a drug delivery system
Researchers observed that some types of cancer like breast and lung caused by accidental erysipelas for hospitalized patients. [ 5 ] Vautier in 1813, first stated the treatment of contagious disease using bacteria and during the treatment of cancer patients, gas gangrene infection was produced by Clostridium perfringens , for tumor deterioration [ 6 – 8 ]. In another episode, a clinician from the United States, William Coley explored live cultures of S. pyogenes , killed extracts of S. pyogenes and Serratia marcescens for the treatment of neck cancer of one of his patients. Subsequent studies showed that various species of bacteria like Bifidobacterium spp. L. monocytogenes, S. typhimurium, E. coli, Clostridium spp. and Mycobacterium bovis were accumulated at the site of tumors. [ 9 – 13 ] However, research revealed that bacteria are not capable to abolish complete tumor but a part of malignant growth was destroyed. Though, a slight portion of the infected tissue may grow to form a large size tumor. For more effective management of cancer and other contagious diseases, bacterial drug delivery is combined with chemotherapies. Bacterial toxins showed an action in contrast to tumors like C. novyi NT. [ 13 , 14 ]
Fungi are not directly used in the drug delivery system but their cell wall component like chitin is used as a carrier. Chitosan-based nanoparticles were prepared by double solvent evaporation technique and used for the delivery of anthracycline and doxorubicin. Hence chitosan-based drug delivery has been emerged due to mucoadhesion, penetration and biodegradation and used as different carrier systems like nanoparticles, microparticles, etc. Algae-based drug delivery system have not been studied much, but algae-based polymer and its blend of components are used as biomaterials.
The research was further extended for targeting the tumor by genetically-engineered bacteria to decrease the toxicity and immunogenicity. Tumor cell death is mostly related to acute toxicity [ 15 , 16 ] and septic shock [ 17 , 18 ] whereas bacteria were manipulated and genetically modified to decrease immunogenicity [ 19 – 21 ]. Genetically modified bacteria used proteins, drugs, enzymes and genes for mitigation of conditions associated with gastrointestinal infection, [ 22 , 23 ] diabetes disorder [ 24 ], carcinoma [ 14 ] and viral infection. [ 25 ] This method shows advantages like site-specific targeting, specific breaking up of tumor cells and interestingly gene-directed enzyme prodrug therapy.
Virosomes are biocompatible, non-toxic and biodegradable carriers and used to deliver various drugs whereas peptides and nucleic acids are delivered by viral cellular envelops. Reconstituted viral envelopes and virosomes may be designed using vaccines as carriers for large molecules like nucleic acids, gene, antibiotics, anticancer agents and steroids. [ 26 ]
Bacterial candidature for drug delivery mechanism
Mobility of bacteria.
The different constituents of bacteria such as flagella and pilli allow them to move [ 27 ] towards oxygen gradient and such property of bacteria is termed as bacterial taxis. [ 28 ] Different classes of bacteria show various taxis behavior like chemotaxis which is the mobility of bacteria in response to change in a chemical environment and phototaxis which involves mobility of bacteria with respect to change in light emission and pH taxis of bacteria. [ 29 ] Such characteristics of bacteria aid them to move towards the specific site. Unlike normal cell, cancer cells of altered chemical composition in comparison to uninfected areas induce chemotaxis property and aid them to travel towards the infected area. Similarly, bacteria are fascinated by the oxygen-deficient site and travelled to tumor area due to hypoxic condition for effective drug delivery system. [ 30 ]
Production of proteins on the site
Current treatment options using conventional dosage form cause uneven distribution of drug in the body. Some bacteria show the capability to deliver proteins only at the desired site without distributing at non-specific sites. These are produced by the bacteria only on-site and acted as a drug for effective delivery by avoiding an unfavorable environment of the stomach and functioned as a drug, prodrug and immune-stimulating agent. [ 31 – 34 ]
Bactofection
The bacterial usage for deliberately introducing naked or purified nucleic acids into eukaryotic cells is called bactofection. The bacterial plasmid combines with the gene of interest, which is absorbed by mammalian cells and transferred to the genetic material of the cell. [ 35 ] For example Bifidobacterium was engineered to deliver a gene to cancer cells. In another promising case, highly specific and productive endostatin gene delivery was reported with species like Bifidobacterium longum in liver diseases. [ 35 – 37 ] Bifidobacterium infantise herpes simplex virus was reported to possess antitumor effect in mice renal cancer cell using thymidine kinase/ganciclovir gene therapy. [ 38 – 40 ]. One of the most important applications of bactofection is the vaccination of DNA to produce action in contrast to several infections and tumors. This vaccine comprises of promoter, antigen and for plasmid wherein desired antigen is encoded by promotor gene of plasmid. [ 41 , 42 ] DNA vaccines were delivered to macrophages by certain bacterial species like L. monocytogenes and L. typhimurium against the pathogens. [ 43 ] Listeria was internalized with bioengineered E. coli to use in breast cancer. [ 44 ]
Stimuli-responsive bacteria
Bacteria shows the capability to react with various stimuli like chemical, pH, light, temperature and also intellect a slight change in their nearby environment. Based on such properties, stimuli-responsive bacteria can be used to target drugs efficiently. [ 45 ]
Light sensitive and light generated transcription
Engineered bacteria revealed light-sensitive ion-channels which allowed to control conventional inducible promoter systems. Bacterial cells are genetically modified in response to light-sensitive ions. A bacterial protein, EL22, a light-oxygen voltage was connected to DNA in blue light and genetically-engineered in response to light-induced transcription. [ 45 ]
Magnetically-responsive bacteria
Certain bacteria synchronize in earth’s magnetic field in the presence of magnetic nanocrystals [ 46 ] and respond to magnetic-stimuli called magnetotactic bacteria. In the presence of magnetic resonance imaging (MRI) [ 47 ], the bacteria may be located and monitored beneath the impact of outer magnetic influence. This idea recommends a novel approach for crafting an accurate therapeutic agent application to infections as well as tumors. [ 48 , 49 ]
Oxygen-driven targeting
Since the cancer cells bear oxygen-deficient condition, anaerobic bacteria like Clostridium and Streptococcus target the tumor [ 50 ]. Clostridium spp . has been modified to transport immunostimulant proteins for upsurging IL-2 facilitates anticancer activity [ 49 ].
Thermo- and pH-responsive delivery
Adherence of Serratia marcescens bacterium to microbeads showed unifacial and bifacial pH- stimulus. In general, cancerous cell is characterized by acidic condition, high temperature and deficit oxygen content [ 51 ]. Due to such distinguished properties between tumor and normal cells, bacteria are able to differentiate and identify tumor cell. These bacteria identify the elevated temperature of the cancer tissue and act like thermo-responsive drug delivery system [ 52 ]. A similar mechanism is observed in pH-responsive drug delivery, bacteria identify the acidic environment and follow pH-responsive mechanism. [ 53 ]
Bacteria for metabolic disorders
Another important application of engineered bacteria for treatment metabolic disorder is obesity. E. coli nissle (EcN) strains were used to express N- acetyltransferase which was obtained from Arabidopis thaliana , this produces N-acyl phosphatidyl ethanolamines (NAPE’s) from small intestine. By incorporating NAPE, EcN in drinking water reduced food consumption and fat gain without undesirable side effects in mice (Table 1 ).
Bacteria and their roles
Advancement in bacteria-based drug delivery system
Bacteria as tumoricidal agents.
Recently, some alive strains of bacteria in the forms of weaken and genetically modified are also used in cancer treatment. Pathogenic bacteria should be avoided to use for conveying of drugs may cause toxicity using non-pathogenic bacteria. These strains show specific and direct actions on the cancer cell or can be used as a vehicle to transport anti-tumor cells at the targeted area. It was revealed that many functions of live, attenuated, nonpathogenic bacteria as an anticancer agent or as a carrier of drugs were also improved [ 54 ]. The precise altered surroundings of solid tumors are recognized and responded by some bacteria to prepare highly target-specific DDS (drug delivery systems). Some of the spores of obligate anaerobes such as Clostridium spp . propagate in the oxygen-deficient region of big tumors because of poor vascularization when given by parenteral route. These properties showed the oncolytic effects on cancer cell because of bacterial growth. It was exposed in animal models as well as in human patients [ 27 ]. Non-infectious strains of Clostridium such as ‘M55’ did not show tumor suppression [ 55 ]. BCG was used for bladder cancer and S. typhimurium derivative and also articulated for cancer therapy [ 54 ]. Certain species of S. choleraesuis , V. cholerae , L. monocytogenes and even E. coli [ 56 ] are currently discovered as anticancer agents .
Bacterium as a vector for drug delivery system
Bacterium like E. coli, S. tryphimurium used as vectors for drug delivery system. The problems associated with bacterial drug delivery systems are the requirement of minimal dose to treat the tumor cell. Dose reduction and safe-effective dose concentration are still under investigation. To avoid such problems, genetically-engineered bacteria are considered as an alternative to provide targeted action and also to convey antitumor drugs at the desired site . [ 54 ]
Bacterial spore as DDS (drug delivery system)
It revealed probiotic properties of oral bacterial therapy were discovered by bacilli spores. These spores after administration by nasal and oral routes exhibited mucosal immune response due to the occurrence of polysaccharides and proteins on the outer surface. The spores of many anaerobic bacteria germinate, multiply, replicate and become lively in some part of the tumor in oxygen lacking areas. (e.g. C. beijerinckii, C.novyi-Non Toxigene and C. histolyticum) [ 57 ]. Anticancer drugs like vinorelbine, docetaxel, mitomycin C and dolastatin-10 [ 14 ] are grouped with spores of C. novyi – NT for better therapeutic action. Genetic engineering increased the antitumor activity of Clostridial spores using prodrug converting enzymes like cytosine deaminase with the capability of converting 5-fluorocytosine (5-FC) to 5- fluorouracil (5-FU). This reduced local dose of antitumor drugs without affecting healthy tissues [ 58 ]. Most of the spores of bacteria such as VNP2009, a genetically engineered species of S. typhimurium for advanced or metastatic solid tumors [ 59 ]. Interleukin 4-PE, Interleukin-4- Pseudomonas exotoxin for brain enteral nervous system tumors [ 60 ] are discovered as antitumor drugs, cytotoxic peptides, and therapeutic proteins and as drug delivery vectors for gene therapy.
Bacterial toxins for tumor therapy
Bacterial toxins mostly used to destroy a tumor, at minimal concentrations modifies cellular processes and regulate cell cycles; cell proliferation, apoptosis and differentiation processes. [ 61 ]
Toxins of bacteria bind to the antigen present on the tumor superficial portion of tumor such as Diphtheria toxin (DT) and Pseudomonas exotoxin suppress protein synthesis. [ 61 ] Some bacterial toxins action was observed because of the antigens which were bound to the antigen existing on the superficial area of cancer cells, such as DT and Pseudomonas exotoxin A identified with catalytically ribosylate EF-2. It causes inhibition of production of protein, destruction of cell and initiation of apoptosis. [ 62 – 64 ] C. perfringens enterotoxin (CPE), an endotoxin, is accountable for gastroenteritis development but its ‘N’ terminal is significant for antitumor activity [ 65 , 66 ]. Various kinds of DT ligands have been investigated for targeting cancer cell (e.g. Interleukin-3, Interleukin-4, Granulocyte Colony Stimulating Factor (GCSF), Transferrin (TF), EGF and vascular endothelial growth factor (VEGF) [ 62 ].
Bacterial cellular envelope
“Bacterial ghosts” are hollow, vacant and non-living envelope of gram-negative bacteria. These develop lysis-tunnel structure inside the covering of live bacterium after regulated expression of cloned bacteriophage gene E. The bacteria are not able to multiply as that of cytoplasm and certain components of DNA are expelled inside the medium but it holds all the structural, immunogenic and bio-adhesive characteristics because of the proteins present on the surface. The cell coverings are safe and may be lyophilized. [ 67 ] many of the drugs, nucleic acids, antigens, and proteins may be formulated with these cellular coverings for non-living drug delivery vehicles because of their unique cellular structures and abilities [ 68 ]. Biotinylated agents were inserted in cytoplasmic membrane of genetically modified Escherichia. coli NM 522 bacterial ghost [ 69 ]. One of the first leak-proof bacterial ghosts concept of E. coli NM 522 was synthesized by Paukneret in 2003. Sealing agents like membrane vesicles were used in the occurrence of Ca ++ ions to avoid the discharge from this bacterial ghost. [ 70 ] Moreover, the formulated BGs of Mannheimia hemolytica with the anticancer agent doxorubicin, when administered systemically, showed a slow release of drug. [ 71 ] It was revealed that bacterial ghost concept of drug delivery can be explored as a convenient agent to transfer of active ingredient to treat ocular superficial disorder [ 72 ].
Bacterial governed enzyme prodrug therapy
The idea of prodrug emerges due to the undesirable side effects of microbial drug delivery systems. The prodrug undergoes biotransformation and forms effective pharmacon molecule in the cancer cell [ 73 ] when administered parenterally. 5-Fluorocytosine (5 FC) is converted to 5-Fluorourocil (5 FU) in presence of enzyme cytosine deaminase (CD) and microbes like C. sporogenes . It has been observed with nitroreductase (NR) which converts the prodrug CB 1954 to a DNA cross-linking agent. Use of active exogenous enzyme for specific delivery to cancerous cell [ 74 , 75 ] was studied with CD expressed in Clostridium acetobutylicum . Another efficacious prodrug enzyme therapy ensured by studies with enzyme cytosine deaminase in hypoxic tumor condition with transfected B. longum by enzyme pBLES100 -S-eCD. [ 76 ]
Bio-hybrid bacteria for drug delivery
Superior levels of performance may be accomplished by combining the microbial cells with non-living materials like microparticles, nanoparticles or microbots. [ 77 ] For instance, the use of distant magnetic steering of a fabricated magnetic microbody may help bacteria to travel towards the target. This is possible when the attachment of fabricated microrobot-specific chemical stimulus and movement of microbes are closed to the intended targeted site [ 78 ]. The delivery of microparticles or nanoparticles of therapeutic agents were targeted by bioengineered bacteria inside the cells as carriers. [ 79 ] They penetrate mammalian cells and transport small molecules, antibodies, therapeutic peptides, and DNA into the cells by employing the bacteria as carriers [ 79 ]. Various pharmaceutically inert materials like polyethylene glycol (PEG) are researched extensively to serve as a carrier due to protein binding resistance property with a bacterial cell for reduction in toxic effect and immunogenicity. [ 75 , 80 ]
Bacteria as immunobiological response modifiers
Immunotherapeutic strategy involves utilization of immune system to kill tumor cells. Enhancement of antigenicity of carcinogenic cells [ 81 ] using bacteria is a new idea of the immuno therapeutic concept. Avogadri et al. showed some fascinating outcomes with an attenuated strain of S. typhimurium invasion of melanoma cells which evicted the occurrence of antigenic elements of bacterial genesis. Surprisingly, fascinating outcomes were seen with intratumor Salmonella injection given to the tumor-bearing mice after vaccinating it with S. typhimurium [ 60 ]. Inflammatory response was triggered by the production of cytokines like IL-6, MI–2, G-CSF and TIMP-1 and by attracting inflammatory cell, and destructing tumor cells after the systemic route of drug delivery of C. novyi-NT spores. Clinical trials in phase I was conducted with spores of C. novyi-NT spores and anti-microtubuli agent [ 81 ]. The use of Lm-LLO-E7 is progressed into clinical trials for cervical cancer therapy [ 82 ] as a cancer immunotherapeutic mediator.
Virus-based drug delivery system
Virotherapy.
One of the applications of biotechnology is virotherapy, where viruses are used as therapeutic agents for the treatment of conditions like cancer and metabolic disorders. Anticancer oncolytic viruses, viral vectors for gene therapy and viral immunotherapy are considered as the three sub-divisions of virotherapy . Cancer patients who were vaccinated earlier and further suffered from non-relevant viral infection showed effectiveness with virotherapy [ 83 ] TNF and interferons are generated by body’s immune system during viral infection; on the other hand, tumor cells are targeted by oncolytic virotherapy. In 1940s and 1950s, the usage of viruses was evaluated by various animal models for the treatment of tumors [ 84 ] whereas human clinical trials were conducted on oncolytic viruses [ 85 ]. An oncolytic virus known as RIGVIR (Riga virus) was developed and registered in 2004 by the Institute of Microbiology in Lativa [ 86 ]. Research studies revealed that the death rate of IB-IIC tumor patients was reduced by 4.3–6.59 folds with RIGVIR. [ 87 ] Researchers from Hebrew University successfully extracted slight different version of Newcastle Virus to target-specific tumor cells. [ 88 ]
Viral immunotherapy
Trovax is an innovative immunotherapeutic treatment by Oxford Biomedia where pox-virus exhibited the tumor antigen 5 T4 instigated immune response to various cancers. [ 89 ]
Virus-like particles (VLPs)
Alike to the virus, VLPs are devoid of viral genetic materials and generally considered as safe . These particles are usually formed from constituents of virus-like viral envelope and capsid-congregate. These particles may be obtained from various virus families like Parvoviridae and Flaviviridae . VLPs can be used as carriers for genes and other therapeutic agents. [ 90 ] In vitro studies revealed that VLPs effectively target cancer cells [ 91 ] because of their higher penetrability and detainment effect. VLPs can be explored as drug delivery tumor imaging because of their accumulation property at the tumor site. [ 92 ]
Immunity against only one strain of microbe is provided by a single vaccine whereas multivalent vaccines, which showed immunity against multiple strains of microorganism. VLPs can be formulated in the form of vaccines. Similar to strong B cell and T-cells reactions, the surface proteins of VLPs also respond for an immune response [ 93 ]. Vaccines for hepatitis and papillomavirus infection used VLPs for FDA approval. [ 94 , 95 ]
Virosomes are restructured viral envelopes are used to deliver vaccines as well as acted as carrier for large molecules like nucleic acids, genes or drugs. Virosomal technology may be explored for drug delivery of drugs like antibiotics, anticancer, and steroids. [ 26 ] The main composition of virosomes is essentially membrane lipids and viral-spike glycoproteins with an empty shell . The first virosomes were prepared by Almeida with liposomes of purified influenza spike proteins [ 96 ]. Subsequently, several viral species including Sendai virus, [ 97 – 99 ] Semliki Forest virus (SFV), [ 100 , 101 ] vesicular stomatitis virus (VSV), [ 102 , 103 ] and Sindbis virus. [ 104 ] were reconstituted in viral envelopes. Proteolytic degradation of therapeutically active substances at less pH can be achieved by virosomal formulation. Because of this unique nature, virosomes may be considered as superior drug delivery compared to liposomes and proteo-liposome carrier systems. [ 105 ]
One of the approaches for formulation and development is that, active pharmaceutical ingredients may be entrapped into the aqueous interior or inside the lipid membrane to facilitate the entry of compounds into the cells. [ 105 ] Virosomes blend with endosome or with the plasma membrane, to deliver these compounds into the cytoplasm of host cell. [ 106 ] This unique feature showed encouraging results and also enhances the application of virosomes technology. The drug delivery of virosomes is achieved by various routes like topical, oral and transdermal. The controlled release of the virus may be achieved by using implants in which they can be incorporated for prolonged delivery. [ 107 ]
Monoclonal antibody-based virus drug delivery
The on-site action of oncolytic medicaments using virosomal vehicle has been demonstrated currently by two distinctive methods: in the initial method, the superficial portion of virosomes carrying an antitumor drug (e.g. doxorubicin) cross-linked to a monoclonal antibody which bind precisely to tumor-related antigens when administered systemically to cancerous tissue whereas in the later method, the virosomes also complexed to ligands and further wrapped the surface receptors on the targeted cells. [ 97 , 105 ] . Different viruses used for drug delivery are listed below:
CCMV (cowpea chlorotic mottle virus)
CCMV (family Bromoviridae ) shows a unique property to undergo a reversible pH-dependent swelling. This property enables them to gather and again separate in-vitro to dislodge the viral genes and then infix the functional species. CCMV is specifically beneficial in encapsulation of negatively charged active materials.
CPMV (cowpea mosaic virus)
CPMV belongs to family chromoviridae and is under research because of its high stability in nanocarriers. This virus is isolated from black-eyed pea plant leaves and stable over extreme conditions of temperature, pH and in many organic solvents.
RCNMV (red clover necrotic mosaic virus)
RCNMV belongs to family Tombusviridae and is useful to carry substances and to change its surface structures. It forms small pores to allow entry of the ions and also enables in packaging.
Routes of administration of microbe-based drug delivery
Depending on the nature of the drug, intended location and ease of administration, the route of microbe-based drug delivery is mentioned below:
Intratumor injection
Zhao et al. found a significant effect of tumor inhibition with intratumoral injection of S. typhimurium without harmful effects like uneven distribution of drug in the body [ 108 ]. Direct injection into the central nervous system is also considered as another method [ 109 , 110 ] where promising results were obtained for tumor-targeting bacteria [ 111 ] with less systemic toxicity and higher efficiency.
Oral administration
Effectiveness for colitis therapy was established by expression of immunosuppressive Inteukin-27 (IL-27) in bioengineered L. lactis when administered orally in mice in comparison to parenteral route. Investigation of type I diabetes by genetically modified GAB-65 and IL- 10 have been reported by oral drug delivery of L. C. difficile diarrhoea vaccination. [ 112 ] Expression of EspB antigen [ 113 ] for hemolytic-uremic syndrome was triggered by E. coli. Ulcerative colitis was treated [ 114 ] with genetically engineered B. longum , expressing α-melanocyte-stimulating hormone, vaccine counter to Hepatitis C virus [ 115 ], and IL-12 anti-inflammatory cytokine by oral route [ 116 ]. S. typhimurium , [ 117 , 118 ] E. coli [ 119 , 120 ] as well as L. casei [ 121 ] are some of the strains of the bacteria were under research via oral route. One of the effective ways to deliver the medicament in cancer patients is intravenous or systemic routes showed higher blood supply than the surrounding tissue [ 122 ]. Some studies also revealed that the in vivo experiments by various strains of bacterium like Bifidobacterium bifidum [ 123 ] and S. typhimurium [ 124 – 126 ] are not affected by systemic administration.

Intranasal injection
Genetically engineered strains of Streptococcus gordonii with antigen expression of Mycobacterium tuberculosis was delivered through intranasal route to sensitize CD 4 + and CD 8 + T-cells and vaccinate [ 127 ] against Neisseira meningitides which is one of the important reasons for meningitis. Administration of Lactobacillus pentosus by intranasal route activates the immune response of the respiratory system.
Advantages of microbe-based drug delivery system
Modified bacteria cause colonization of intestinal lumen which specifically conveys protective cytokines, growth factors and competitive inhibitors at the desired site, which also help in penetration of recombinant molecules in the inflamed area.
Carriers used for target-specific drug delivery avoid systemic toxicity, minimize host-immune responses to vectors and maximize local therapeutic concentrations of the actives.
Economical treatment for chronic diseases like IBD can be achieved by expanding the idea of microbial drug delivery, by replication of the microbes specifically to colonize in the intestine.
To avoid the resistance of tumor cells to the anticancer agents, the bacterial therapy combined with cytotoxic agents are explored in the form of bacterial cellular envelope encapsulated with doxorubicin for the treatment of breast cancer.
Disadvantages of microbe-based drug delivery system
Dose associated toxicity and severity of systemic infection need to be explained
Combination of chemotherapy is suggested with bacteria-based delivery as the microbes are not utilized key portions of the cancer tissue (i.e. partial tumor lysis) [ 54 ]
DNA mutation is another possibility which may letdown the therapy.
Safety and regulatory concerns are complicated.
Use of bacteria in drug delivery
Clostridium novyi NT is under investigation for its unique properties. This bacterium shows the capability to permeate and destruct the tissues, and also boost the host immune system. The extended application includes the treatment of hypoxic tumor conditions.
Lactococcus lactis , another class of bacteria is examined for inflammation related to colitis. TNF is responsible for these inflammatory symptoms of colitis. IL-10 is an agent that balances and decreases the inflammation related to colitis. Lactococcus is engineered in a specific way to produce IL-10.
Toxicity associated with microbe-based DDS
In application of microbes-based drug delivery, the use of live bacteria showed many hazardous reactions due to discharge of intracellular contents, endotoxins of microbes and chemicals like induction of inflammatory responses.
The main problem associated with microbial based drug-delivery systems is to alter dose-dependent toxicity. The foremost complications associated with microbial DDS which may change the therapeutic efficacy. Hypersensitivity reactions and quick removal of bacteria [ 128 ] were reported and dependent on the immune response at higher bacterial concentrations. In spite of the removal of toxic genes [ 129 ], residual toxicity has been observed. Therefore, the bacteria must not interfere with the patient’s immune response, easily removed after treatment and in poor host response. [ 130 ] For a complete summary on toxicity research, different administrative recommendations such as ‘Guidance for industry concentrations for developmental toxicity studies for preventive and therapeutic vaccines for infectious disease indications’ by US FDA [ 131 ] and ICH guideline: Guidance on testing of genotoxicity studies and interpretation of data for pharmaceuticals intended for human use [ 132 ] and immunotoxicity studies for human pharmaceuticals [ 132 ] are approved.
Regulatory concerns
Drug delivery using microbes is a diversified system compared to conventional route due to either live or vegetative state and are capable of self-propagating within tumor cells. The biggest challenge with microbial drug delivery is its distinctive regulatory requirements with additional safety, toxicity and manufacturing technology. Guidelines and recommendations of genetic engineering techniques like recombinant DNA technologies used for these agents are provided by regulatory authorities like USFDA and the Office of Biotechnology Activities (OBA) at the National Institutes of Health [ 133 ] has carried out a detailed analysis in this regard and instructive guidelines by USFDA like [ 134 ] are also available. “Guidance for Industry: determining the need for and content of environmental assessments for gene therapies, vectored vaccines, and related recombinant viral or microbial products”.
Conclusions
Microbe-based drug delivery has become one of the promising delivery systems for many diseases like cancer, inflammatory bowel diseases. The main problem associated with conventional drug delivery system is the resistance developed by tumor cells. So as to overcome this problem, microbial drug therapy in combination with an anti-cancer agent play important role in drug delivery system. Toxins and spores are the bacterial products and helpful candidates to treat carcinogenic cells. Moreover, other strategies to treat tumor cells include bacterial ghosts, microbots and bactofection. Even if microbial therapy shows favorable applications, toxicity, dose and therapeutic efficacy still remain a problem. Furthermore, advance development and research in this field is required. Another novel approach is virotherapy, which includes using the virus, virosomes, viral particles, to convey molecules, nucleic acids, biological actives, etc. However, some parameters need to be studied such as toxicity and compatibility of the virus with molecules.
Bacteria show promising results because of their exploited properties like penetration in the tumor, increase in immunity, etc. Bacteria in drug delivery are extensively under research and achieved success in preclinical trails as well as in clinical trials. Other than bacteria, viruses are equally proved to be an interesting candidate for drug delivery due to accumulation in tumor and tumor imaging. A novel approach of microbes-based drug delivery with reduced toxicity and side effects will surely be futuristic advanced carrier to active improve patient’s health.
Authors contributions
Dr. Pravin Shende is involved in constructing, planning and organizing the manuscript.
Ms. Vasavi Basarkar is involved in literature search and writing of manuscript.
Compliance with ethical standards
Conflict of interest.
Authors declare no conflict of interest.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Chang WW, Lee C. Salmonella as an innovative therapeutic antitumor agent. J Mol Sci. 2014;15:14546–14544. doi: 10.3390/ijms150814546. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Bhaskar M, Sanib B. Virosomes: a novel strategy for drug Deliveryand targeting. Bio-Pharm International Supplements. 2011;24:12–13. [ Google Scholar ]
- 3. Kumar A, Kumar A.V., Why Chitosan? From properties to perspective ofmucosal drug delivery, J Biological Macromolecules. 2016; (S0141–8130) (16) 30465–2. [ DOI ] [ PubMed ]
- 4. Yin L. Bacillus spore-based oral carriers loading curcumin for the therapy of colon cancer. J Control Release. 2017;S0168-3659(17):31079–9. [ DOI ] [ PubMed ]
- 5. Nauts H, The beneficial effects of bacterial infections on host resistance to cancer: End result in 449 cases, Cancer research institute monograph no. 8, New York, USA; 1980; (2).
- 6. Barbe S, Mellaert V, Anne J. The use of clostridial spores for cancer treatment. J Appl Microbiol. 2006;101(3):571–578. doi: 10.1111/j.1365-2672.2006.02886.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Richardson MA, Ramirez T, Russell NC, Moye LA. Coley toxins immunotherapy: a retrospective review. AlternTher Health Med. 1999;5(3):42. [ PubMed ] [ Google Scholar ]
- 8. Zacharski LR, Sukhatme VP. Coley’s toxin revisited: immunotherapy or plasminogen activator therapy of cancer. J Thromb Haemost. 2005;3(3):424–427. doi: 10.1111/j.1538-7836.2005.01110.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Gravekamp C, Paterson Y. Harneeing Listeria monocytogenes to target tumors. Cancer Biol Ther. 2010;9:257–265. doi: 10.4161/cbt.9.4.11216. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Forbes NS. Perspectives. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Hoffman HR, Zhao M. Methods for the development of tumor targeting bacteria. Expert Opinion. J Drug Discov. 2014;(9):741–50. [ DOI ] [ PubMed ]
- 12. Minton NP. Clostridia in cancer therapy. Nat Re Microbiol. 2003;1:237–242. doi: 10.1038/nrmicro777. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Carswell EP, Old LJ, Kassel RL, Green S. Williamson, an endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Dang LH, Bettegowda C, Huso DL, Kinzler K, Vogelstein WB. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci. 2001;98(26):15155–15160. doi: 10.1073/pnas.251543698. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Gericke D, Engelbart K. Oncolysis by clostridia. II. Experiments on a tumor spectrum, 1208. Cancer Res. 1964;24:217–221. [ PubMed ] [ Google Scholar ]
- 16. Thiele EH, Arison RN, Boxer G. E., Oncolysis by clostridia. IV. Effect of nonpathogenic 1210 clostridial spores. Cancer Res. 1964;24:234–238. [ PubMed ] [ Google Scholar ]
- 17. Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992;268:3452–3455. [ PubMed ] [ Google Scholar ]
- 18. Dinarello CA, Gelfand JA, Wolff SM. Anticytokine strategies in the treatment of the 1214 systemic inflammatory response syndrome. JAMA. 1993;269:1829–1835. [ PubMed ] [ Google Scholar ]
- 19. Jr Somerville JE, Cassiano LBB, Cunningham MD, Darveau RP. A novel 1216 Escherichia coli lipid a mutant that produces an anti-inflammatory lipopolysaccharide, 1217 J. Clin Investig. 1996;97:359–365. doi: 10.1172/JCI118423. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Khan S.A., Everest, P. Servos S, Foxwell N, Zahringer U, et al. 1219 Dougan, I.G. Charles, D.J. Maskell, A lethal role for lipid, A in Salmonella infections, Mol.1220 Microbiol. 1998; 29, 571–579. [ DOI ] [ PubMed ]
- 21. Low K. Brooks, Ittensohn Martina, Le Trung, Platt James, Sodi Stefano, Amoss Max, Ash Olivia, Carmichael Ellen, Chakraborty Ashok, Fischer Jessica, Lin Stanley L., Luo Xiang, Miller Samuel I., Zheng Li-mou, King Ivan, Pawelek John M., Bermudes* David. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nature Biotechnology. 1999;17(1):37–41. doi: 10.1038/5205. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Foligne Benoit, Dessein Rodrigue, Marceau Michael, Poiret Sabine, Chamaillard Mathias, Pot Bruno, Simonet Michel, Daniel Catherine. Prevention and Treatment of Colitis With Lactococcus lactis Secreting the Immunomodulatory Yersinia LcrV Protein. Gastroenterology. 2007;133(3):862–874. doi: 10.1053/j.gastro.2007.06.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Steidler L. Treatment of Murine Colitis by Lactococcus lactis Secreting Interleukin-10. Science. 2000;289(5483):1352–1355. doi: 10.1126/science.289.5483.1352. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes. 2015;64(5):1794–1803. doi: 10.2337/db14-0635. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Liu X., Lagenaur L. A., Simpson D. A., Essenmacher K. P., Frazier-Parker C. L., Liu Y., Tsai D., Rao S. S., Hamer D. H., Parks T. P., Lee P. P., Xu Q. Engineered Vaginal Lactobacillus Strain for Mucosal Delivery of the Human Immunodeficiency Virus Inhibitor Cyanovirin-N. Antimicrobial Agents and Chemotherapy. 2006;50(10):3250–3259. doi: 10.1128/AAC.00493-06. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Mazumder B., Bhattacharya S., Virosomes: a novel strategy for drug delivery and targeting. The science and business of biopharmaceuticals, Biochem Pharmacol. 2001; Jan.02, 24.
- 27. Paton Adrienne W., Morona Renato, Paton James C. Bioengineered microbes in disease therapy. Trends in Molecular Medicine. 2012;18(7):417–425. doi: 10.1016/j.molmed.2012.05.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Goldstein Richard A., Soyer Orkun S. Evolution of Taxis Responses in Virtual Bacteria: Non-Adaptive Dynamics. PLoS Computational Biology. 2008;4(5):e1000084. doi: 10.1371/journal.pcbi.1000084. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Taylor BL, Zhulin IB, Johnson MS. Aerotaxis and other energy-sensing behavior in bacteria. Annu Rev Microbiol. 1999;53:103–128. doi: 10.1146/annurev.micro.53.1.103. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Taniguchi S., Shimatani Y., Fujimori M., Tumor-targeting therapy using gene-engineered anaerobic-nonpathogenic Bifidobacterium longum , Methods Mol Biol 2016; 1409,1375 49–60. [ DOI ] [ PubMed ]
- 31. Seavey M. M., Pan Z.-K., Maciag P. C., Wallecha A., Rivera S., Paterson Y., Shahabi V. A Novel Human Her-2/neu Chimeric Molecule Expressed by Listeria monocytogenes Can Elicit Potent HLA-A2 Restricted CD8-positive T cell Responses and Impact the Growth and Spread of Her-2/neu-positive Breast Tumors. Clinical Cancer Research. 2009;15(3):924–932. doi: 10.1158/1078-0432.CCR-08-2283. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Theys J, Pennington O, Dubois L, Anlezark G, Vaughan T, Mengesha A, Landuyt W, Anné J, Burke P J, Dûrre P, Wouters B G, Minton N P, Lambin P. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo. British Journal of Cancer. 2006;95(9):1212–1219. doi: 10.1038/sj.bjc.6603367. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Du Z.Q., Wang J.Y. A novel lumazine synthase molecule from Brucella significantly promotes the immune-stimulation effects of antigenic protein. Genetics and Molecular Research. 2015;14(4):13084–13095. doi: 10.4238/2015.October.26.4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Friend DR, Chang GW. A colon-specific drug-delivery system based on drug glycosides and the glycosidases of colonic bacteria. J Med Chem. 1984;27:261–266. doi: 10.1021/jm00369a005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Fu GF, Li X, Hou YY, Fan YR, Liu WH, Xu GX. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther. 2005;12:133–140. doi: 10.1038/sj.cgt.7700758. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Yazawa K, et al. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat. 2001;66:165–170. doi: 10.1023/a:1010644217648. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Lee Byeongdu, Lo Chieh-Tsung, Thiyagarajan P., Winans Randall E., Li Xuefa, Niu Zhongwei, Wang Qian. Effect of Interfacial Interaction on the Cross-Sectional Morphology of Tobacco Mosaic Virus Using GISAXS. Langmuir. 2007;23(22):11157–11163. doi: 10.1021/la7009989. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Xiao Xiao, Jin Ren, Li Jiang, Bei Yu, Wei Tang. The Antitumor Effect of Suicide Gene Therapy Using Bifidobacterium infantis–mediated Herpes Simplex Virus Thymidine Kinase/Ganciclovir in a Nude Mice Model of Renal Cell Carcinoma. Urology. 2014;84(4):982.e15-982.e20. doi: 10.1016/j.urology.2014.05.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Jiang L. Proteomic analysis of bladder cancer by iTRAQ after Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene treatment. Biol Chem. 2013;394:1333–1342. doi: 10.1515/hsz-2013-0201. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Yin X. Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancer. Cancer Gene Ther. 2013;20:77–81. doi: 10.1038/cgt.2012.86. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Yin X, Yu B, Tang Z, He B, Xiao X. Bifidobacterium infantis-mediated HSV-TK\ GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancer. Cancer Gene Ther. 2013;20:77–81. doi: 10.1038/cgt.2012.86. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Okuda K, Wada Y, Shimada M. Recent developments in preclinical DNA vaccination. Vaccines (Basel) 2014;2:89–106. doi: 10.3390/vaccines2010089. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Gentschev Ivaylo, Dietrich Guido, Spreng Simone, Kolb-Mäurer Annette, Brinkmann Volker, Grode Leander, Hess Jürgen, Kaufmann Stefan H.E., Goebel Werner. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine. 2001;19(17-19):2621–2628. doi: 10.1016/s0264-410x(00)00502-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Radford KJ, Higgins DE, Pasquini S, Cheadle EJ, Carta LA. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation: Application to cancer immunotherapy. Gene Ther. 2002;9:1455–1463. doi: 10.1038/sj.gt.3301812. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Storz G., Hengge, R., Bacterial Stress Responses, 2nd ed. American Society for Microbiology Press. 2010.
- 46. Faivre D. Magnetotactic bacteria and magnetosomes. Chem Rev. 2008;108:4875–4898. doi: 10.1021/cr078258w. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Felfoul Ouajdi, Martel Sylvain. Assessment of navigation control strategy for magnetotactic bacteria in microchannel: toward targeting solid tumors. Biomedical Microdevices. 2013;15(6):1015–1024. doi: 10.1007/s10544-013-9794-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Martel S. Bacterial microsystems and microrobots. Biomed Microdevices. 2012;14:1033–1045. doi: 10.1007/s10544-012-9696-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Chen CY, Song T. Construction of a microrobot system using magnetotactic bacteria for the separation of staphylococcus aureus. Biomed Microdevices. 2014;16:761–770. doi: 10.1007/s10544-014-9880-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Brown J. Martin, Wilson William R. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J Nucl Med. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Kluger M., Rothenburg B. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979;203(4378):374–376. doi: 10.1126/science.760197. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Zhuan, G.J., Wright Carlsen R., Sitti M., pH-taxis of biohybrid microsystems, Sci. Report. 2015; 5, 11403. [ DOI ] [ PMC free article ] [ PubMed ]
- 54. Patyar S, Joshi R, Byrav DS, Das P. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17(1):21. doi: 10.1186/1423-0127-17-21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Carey R.W., Holland J.F., Whang H.Y., Neter E., Bryant B. Clostridial oncolysis in man. European Journal of Cancer (1965) 1967;3(1):37–46. [ Google Scholar ]
- 56. Bermudes D, Zheng L, King IC. Live bacteria as anticancer agents and tumor-selective protein delivery vectors. CurrOpin Drug DiscovDevel. 2002;5(2):194–199. [ PubMed ] [ Google Scholar ]
- 57. Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [ PubMed ] [ Google Scholar ]
- 58. Liu S, Minton N, Giaccia A, Brown J. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9(4):291–296. doi: 10.1038/sj.gt.3301659. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. King I, Itterson M, Bermudes D. Tumor-targeted Salmonella typhimurium overexpressing cytosine deaminase: a novel, tumor-selective therapy. Methods Mol Biol. 2009;542:649–659. doi: 10.1007/978-1-59745-561-9_33. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Avogadri Francesca, Martinoli Chiara, Petrovska Liljana, Chiodoni Claudia, Transidico Pietro, Bronte Vincenzo, Longhi Renato, Colombo Mario P., Dougan Gordon, Rescigno Maria. Cancer Immunotherapy Based on Killing ofSalmonella-Infected Tumor Cells. Cancer Research. 2005;65(9):3920–3927. doi: 10.1158/0008-5472.CAN-04-3002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Sabzehali F, Azimi H, Goudarzi M. Bacteria as a vehicle in cancer therapy and drug delivery. J of Paramedical Sciences. 2017; (8), 1 52–59.
- 62. Frankel Arthur, Rossi Patrick, Kuzel Timothy, Foss Francine. Diphtheria Fusion Protein Therapy of Chemoresistant Malignancies. Current Cancer Drug Targets. 2002;2(1):19–36. doi: 10.2174/1568009023333944. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Falnes PO, Ariansen S, Sandvig K, Olsnes S. Requirement for prolonged action in the cytosol for optimal protein synthesis inhibition by diphtheria toxin. J Biol Chem. 2005;275(6):4363–4368. doi: 10.1074/jbc.275.6.4363. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Pastan I., Targeted therapy of cancer with recombinant Immunotoxins Bio chimicaet Biphysica Acta (BBA)-Rev Cancer. 1997; 1333(2) C1-C6. [ DOI ] [ PubMed ]
- 65. Kokai JF, Mcclane BA. Determination of functional regions of Clostridium perfringens enterotoxin through deletion analysis. Clin Infect Dis. 1997;25:S165–S167. doi: 10.1086/516246. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Kokai JF, Benton K, Wieckowski EU, Mcclane BA. Identification of a Clostridium perfringens enterotoxin region required for large complex formation and cytotoxicity by random mutagenesis. Infect Immun. 1999;67:5634–5641. doi: 10.1128/iai.67.11.5634-5641.1999. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: production and applications. Bioengineered Bugs. 2010;1:326–336. doi: 10.4161/bbug.1.5.12540. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Tabrizi Chakameh Azimpour, Walcher Petra, Mayr Ulrike Beate, Stiedl Thomas, Binder Matthias, McGrath John, Lubitz Werner. Bacterial ghosts – biological particles as delivery systems for antigens, nucleic acids and drugs. Current Opinion in Biotechnology. 2004;15(6):530–537. doi: 10.1016/j.copbio.2004.10.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Huter V, Szostak MP, Prethaler GJ. Bacterial ghosts as drug carrier and targeting vehicles. J Control Release. 1999;61:51–63. doi: 10.1016/s0168-3659(99)00099-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Paukner S, Kohl G, Jalava K. Lubitz W, sealed bacterial ghosts—novel targeting vehicles for advanced drug delivery of water-soluble substances. J Drug Target. 2003;11:151–161. doi: 10.1080/10611860310001593366. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Paukner S, Kohl G. Lubitz W, bacterial ghosts as novel advanced drug delivery systems: anti proliferative activity of loaded doxorubicin in human Caco-2 cells. J Control Release. 2004;94:63–74. doi: 10.1016/j.jconrel.2003.09.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Stein Elisabeth, Inic-Kanada Aleksandra, Belij Sandra, Montanaro Jacqueline, Bintner Nora, Schlacher Simone, Mayr Ulrike Beate, Lubitz Werner, Stojanovic Marijana, Najdenski Hristo, Barisani-Asenbauer Talin. In Vitro and In Vivo Uptake Study ofEscherichia coliNissle 1917 Bacterial Ghosts: Cell-Based Delivery System to Target Ocular Surface Diseases. Investigative Opthalmology & Visual Science. 2013;54(9):6326. doi: 10.1167/iovs.13-12044. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Mengesha, In: Clostridia: Molecular Biology in the Post-genomic Era. Bruggemann H, Gottschalk G, editor. Caister Academic Press; Clostridia in Anti-tumor Therapy. 2009.
- 74. Theys Jan, Landuyt Willy, Nuyts Sandra, Van Mellaert Lieve, van Oosterom Allan, Lambin Philippe, Anné Jozef. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Therapy. 2001;8(4):294–297. doi: 10.1038/sj.cgt.7700303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Rabanel JM, Hildgen P, Banquy X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J Control Release. 2014;185:71–87. doi: 10.1016/j.jconrel.2014.04.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Fujimori M. AmanoJ. Taniguchi S The genus Bifidobacterium for cancer gene therapy CurrOpin Drug Discov Devel. 2003;5:200–203. [ PubMed ] [ Google Scholar ]
- 77. Carlsen Rika Wright, Sitti Metin. Bio-Hybrid Cell-Based Actuators for Microsystems. Small. 2014;10(19):3831–3851. doi: 10.1002/smll.201400384. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Kim D, Liu A. Diller E, Sitti M, chemotactic steering of bacteria propelled microbeads, 1717 biomed. Microdevices. 2012;14:1009–1017. doi: 10.1007/s10544-012-9701-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K. Bacteria-mediateddelivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007;2:441–449. doi: 10.1038/nnano.2007.149. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Kolate A. BaradiaD, PatilS, VhoraI., PEG –a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81. doi: 10.1016/j.jconrel.2014.06.046. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Xu J, et al. Combination of immunotherapy with anaerobic bacteria for immunogene therapy of solid tumours. Gene TherMol Biol. 2009;13:36–52. [ Google Scholar ]
- 82. Wood Laurence M., Guirnalda Patrick D., Seavey Matthew M., Paterson Yvonne. Cancer immunotherapy using Listeria monocytogenes and listerial virulence factors. Immunologic Research. 2008;42(1-3):233–245. doi: 10.1007/s12026-008-8087-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Kelly RE. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Moore AE. The destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer. 1949;2(3):525–534. doi: 10.1002/1097-0142(194905)2:3<525::aid-cncr2820020317>3.0.co;2-o. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Clinical virotherapy: four historically significant clinical trials.
- 86. Simona D, Strēle L, Proboka G, Auziņš J, Pēteris A, Björn J, et al. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015;25(5):421–6. [ DOI ] [ PMC free article ] [ PubMed ]
- 87. Viruses: The new cancer hunters. Isra Cast (News article). March 1, 2016.
- 88. Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo- controlled phase III study. Clin Cancer Res. 2010;16(22):5539–5547. doi: 10.1158/1078-0432.CCR-10-2082. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Bayer ME, Blumberg BS, Werner B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down's syndrome and hepatitis. Nature. 1968;218(5146):1057–1059. doi: 10.1038/2181057a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Petry H, Goldmann C, Ast O. Lüke W, "the use of virus-like particles for gene transfer". Curr Opin Mol Ther. 2003;5(5):524–528. [ PubMed ] [ Google Scholar ]
- 91. Galaway FA, Stockley PG. MS2 virus like particles: A robust, semisynthetic targeted drug delivery platform. Mol Pharm. 2013;10:59–68. doi: 10.1021/mp3003368. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Kovacs EW, et al. Dual-surface-modified bacteriophage MS2 as an ideal scaffold for a viral capsid-based drug delivery system. Bioconjug Chem. 2007;18:1140–1147. doi: 10.1021/bc070006e. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Akahata Wataru, Yang Zhi-Yong, Andersen Hanne, Sun Siyang, Holdaway Heather A, Kong Wing-Pui, Lewis Mark G, Higgs Stephen, Rossmann Michael G, Rao Srinivas, Nabel Gary J. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nature Medicine. 2010;16(3):334–338. doi: 10.1038/nm.2105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Zhang Xiao, Xin Lu, Li Shaowei, Fang Mujin, Zhang Jun, Xia Ningshao, Zhao Qinjian. Lessons learned from successful human vaccines: Delineating key epitopes by dissecting the capsid proteins. Human Vaccines & Immunotherapeutics. 2015;11(5):1277–1292. doi: 10.1080/21645515.2015.1016675. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Shende P, Waghchaure M. Combined vaccines for prophylaxis of infectious conditions. Artif Cells Nanomed Biotechnol. 2019;47(1):696–705. doi: 10.1080/21691401.2019.1576709. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Almeida JD, Brand CM, Edwards DC. T.D. Heath, Formation of virosomes from influenza subunits and liposomes. Lancet. 1975;2:899–901. doi: 10.1016/s0140-6736(75)92130-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Bagai S, Puri A. Hemagglutinin- neuraminidase enhances F protein-mediated membrane fusion of reconstituted Sendai virus envelopes with cells. J Virol. 1993;67:3312–3318. doi: 10.1128/jvi.67.6.3312-3318.1993. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Uchida T, Kim J. Reconstitution of lipid vesicles associated with HVJ (Sendai virus) spikes. Purification and some properties of vesicles containing nontoxic fragment a of diphtheria toxin. J Cell Biol. 1979;80:10–20. doi: 10.1083/jcb.80.1.10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Vainstein A, Hershkovitz M, Israel S, Rabin S, Loyter A. A new method for reconstitution of highly fusogenic Sendai virus envelopes. Biochim Biophys Acta. 1984;773:181–188. doi: 10.1016/0005-2736(84)90081-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Helenius A, Sarvas M, Simons K. Asymmetric and symmetric membrane reconstitution by detergent elimination. Studies with Semliki-Forest-virus spike glycoprotein and penicillinase from the membrane of Bacillus licheniformis Eur J Biochem. 1981;116:27–35. doi: 10.1111/j.1432-1033.1981.tb05296.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Helenius A, Fries E, Kartenbeck J. Reconstitution of Semliki Forest virus membrane. J Cell Biol. 1977;75:866–880. doi: 10.1083/jcb.75.3.866. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Metsikkö K, Simons K. Reconstitution of the fusogenicactivity of vesicular stomatitis virus. EMBO J. 1986;5:3429–3435. doi: 10.1002/j.1460-2075.1986.tb04665.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Petri WA, Wagner RR. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979;(254):4313–6. [ PubMed ]
- 104. Scheule RK. Novel preparation of functional Sindbis virosomes. Biochem. 1986;25:4223–4232. doi: 10.1021/bi00363a009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Cusi MG. Applications of influenza virosomes as a delivery system. Human Vaccines. 2006;2:1–7. doi: 10.4161/hv.2.1.2494. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. DaemenTde Mare A, Bungener L, de Jonge J, Huckriede A, Wilschut J. Virosomes for antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:451–463. doi: 10.1016/j.addr.2004.09.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Felnerova D, Viret JF, Glück R, Moser C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:518–529. doi: 10.1016/j.copbio.2004.10.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Zhao S, Penman M, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Rosenberg GA. Neurological diseases in relation to the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32:1139–1151. doi: 10.1038/jcbfm.2011.197. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Van Sorge N. M, Doran K.S., Defense at the border: the blood–brain barrier versus bacterial foreigners, Future Microbiol 7, 2012; 383–394. [ DOI ] [ PMC free article ] [ PubMed ]
- 111. Zwagerman Nathan T., Friedlander Robert M., Monaco Edward A. Intratumoral Clostridium novyi as a Potential Treatment for Solid Necrotic Brain Tumors. Neurosurgery. 2014;75(6):N17–N18. doi: 10.1227/01.neu.0000457197.94533.68. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Guo Shanguang, Yan Weiwei, McDonough Sean P., Lin Nengfeng, Wu Katherine J., He Hongxuan, Xiang Hua, Yang Maosheng, Moreira Maira Aparecida S., Chang Yung-Fu. The recombinant Lactococcus lactis oral vaccine induces protection against C. difficile spore challenge in a mouse model. Vaccine. 2015;33(13):1586–1595. doi: 10.1016/j.vaccine.2015.02.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Ahmed B, Loos M, Vanrompay D, Cox E. Oral immunization with Lactococcus lactis-expressing EspB induces protective immune responses against Escherichia coli O157:H7 in a murine model of colonization. Vaccine. 2014;32:3909–3916. doi: 10.1016/j.vaccine.2014.05.054. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Wei P, et al. Oral delivery of Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to combat ulcerative colitis. J Med Microbiol. 2016;65(2):160–168. doi: 10.1099/jmm.0.000197. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Takei Saki, Omoto Chika, Kitagawa Koichi, Morishita Naoya, Katayama Takane, Shigemura Katsumi, Fujisawa Masato, Kawabata Masato, Hotta Hak, Shirakawa Toshiro. Oral administration of genetically modified Bifidobacterium displaying HCV-NS3 multi-epitope fusion protein could induce an HCV-NS3-specific systemic immune response in mice. Vaccine. 2014;32(25):3066–3074. doi: 10.1016/j.vaccine.2014.03.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Yu Zhijian, Huang Zhen, Sao Chongwen, Huang Yuanjian, Zhang Fan, Yang Jin, Lian Jie, Zeng Zhongming, Luo Wenshen, Zeng Weiseng, Deng Qiwen. Bifidobacterium as an oral delivery carrier of interleukin-12 for the treatment of Coxsackie virus B3-induced myocarditis in the Balb/c mice. International Immunopharmacology. 2012;12(1):125–130. doi: 10.1016/j.intimp.2011.10.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Ning JF, Zhu W, Xu JP, Zheng CY, Meng XL. Oral delivery of DNA vaccine encoding VP28 against white spot syndrome virus in crayfish by attenuated Salmonella typhimurium. Vaccine. 2009;27:1127–1135. doi: 10.1016/j.vaccine.2008.11.075. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Chen Guo, Wei Dong-Ping, Jia Li-Jun, Tang Bo, Shu Luan, Zhang Kui, Xu Yun, Gao Jing, Huang Xiao-Feng, Jiang Wen-Hui, Hu Qin-Gang, Huang Yan, Wu Qiang, Sun Zhi-Hua, Zhang Jian-Fa, Hua Zi-Chun. Oral delivery of tumor-targetingSalmonellaexhibits promising therapeutic efficacy and low toxicity. Cancer Science. 2009;100(12):2437–2443. doi: 10.1111/j.1349-7006.2009.01337.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 119. Castagliuolo I, Beggiao E, Brun P, Barzon L, Goussard S, Manganelli R, Grillot-Courvalin C, Palù G. Engineered E. coli delivers therapeutic genes to the colonic mucosa. Gene Therapy. 2005;12(13):1070–1078. doi: 10.1038/sj.gt.3302493. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Grillot-Courvalin C, et al Fruehauf, Development of a therapeutic RNAi delivery system using nonpathogenic bacteria expressing inv and hly: trans kingdom RNA interference (tkRNAi), Hum. Gene Ther. 2009; 20, 670.
- 121. Ivory K., Chambers S. J., Pin C., Prieto E., Arqués J. L., Nicoletti C. Oral delivery ofLactobacillus caseiShirota modifies allergen-induced immune responses in allergic rhinitis. Clinical & Experimental Allergy. 2008;38(8):1282–1289. doi: 10.1111/j.1365-2222.2008.03025.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Lee CH. Engineering bacteria toward tumor targeting for cancer treatment: current state and perspectives. Appl Microbiol Biotechnol. 2012;93:517–523. doi: 10.1007/s00253-011-3695-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Ganai S, Arenas R B, Sauer J P, Bentley B, Forbes N S. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Therapy. 2011;18(7):457–466. doi: 10.1038/cgt.2011.10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Loeffler M. Et al reed, inhibition of tumor growth using Salmonella expressing Fas ligand. J Natl Cancer Inst. 2008;100:1113–1116. doi: 10.1093/jnci/djn205. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 126. Yam Clinton, Zhao Ming, Hayashi Katsuhiro, Ma Huaiyu, Kishimoto Hiroyuki, McElroy Michele, Bouvet Michael, Hoffman Robert M. Monotherapy with a Tumor-Targeting Mutant of S. typhimurium Inhibits Liver Metastasis in a Mouse Model of Pancreatic Cancer. Journal of Surgical Research. 2010;164(2):248–255. doi: 10.1016/j.jss.2009.02.023. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Ciabattini, A. et al, Primary activation of antigen- specific naive CD4 (+) and CD8 (+) T cells following intranasal vaccination with recombinant bacteria, Infect. Immun. 76. 2008; 5817–5825. [ DOI ] [ PMC free article ] [ PubMed ]
- 128. Palffy F. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Ther. 2006;13:101–105. doi: 10.1038/sj.gt.3302635. [ DOI ] [ PubMed ] [ Google Scholar ]
- 129. Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [ PubMed ] [ Google Scholar ]
- 130. Hosseinidoust Z. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44. doi: 10.1016/j.addr.2016.09.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Guidance for Industry: Considerations for developmental toxicity studies for preventive and therapeutic vaccines for infectious disease indications. 2006.
- 132. ICH guideline on Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use. 2011. [ PubMed ]
- 133. Husain S R, Han J, Au P, Shannon K, Puri R K. Gene therapy for cancer: regulatory considerations for approval. Cancer Gene Therapy. 2015;22(12):554–563. doi: 10.1038/cgt.2015.58. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Guidance for Industry: Design and Analysis of Shedding Studies for Virus or Bacteria-based Gene Therapy and Oncolytic Products. 2015; (73).
- View on publisher site
- PDF (465.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Open access
- Published: 06 June 2020
Recent advances in polymeric drug delivery systems
- Yong Kiel Sung ORCID: orcid.org/0000-0001-7009-8822 1 , 2 &
- Sung Wan Kim 2
Biomaterials Research volume 24 , Article number: 12 ( 2020 ) Cite this article
61k Accesses
453 Citations
8 Altmetric
Metrics details
Polymeric drug delivery systems have been achieved great development in the last two decades. Polymeric drug delivery has defined as a formulation or a device that enables the introduction of a therapeutic substance into the body. Biodegradable and bio-reducible polymers make the magic possible choice for lot of new drug delivery systems. The future prospects of the research for practical applications has required for the development in the field.
Natural polymers such as arginine, chitosan, dextrin, polysaccharides, poly (glycolic acid), poly (lactic acid), and hyaluronic acid have been treated for polymeric drug delivery systems. Synthetic polymers such as poly (2-hydroxyethyl methacrylate), poly(N-isopropyl acrylamide)s, poly(ethylenimine)s, dendritic polymers, biodegradable and bio-absorbable polymers have been also discussed for polymeric drug delivery. Targeting polymeric drug delivery, biomimetic and bio-related polymeric systems, and drug-free macromolecular therapeutics have also treated for polymeric drug delivery. In polymeric gene delivery systems, virial vectors and non-virial vectors for gene delivery have briefly analyzed. The systems of non-virial vectors for gene delivery are polyethylenimine derivatives, polyethylenimine copolymers, and polyethylenimine conjugated bio-reducible polymers, and the systems of virial vectors are DNA conjugates and RNA conjugates for gene delivery.
The development of polymeric drug delivery systems that have based on natural and synthetic polymers are rapidly emerging to pharmaceutical fields. The fruitful progresses have made in the application of biocompatible and bio-related copolymers and dendrimers to cancer treatment, including their use as delivery systems for potent anticancer drugs. Combining perspectives from the synthetic and biological fields will provide a new paradigm for the design of polymeric drug and gene delivery systems.
Introduction
The research for polymeric drug delivery has been progressed for a long time since 1980’s [ 1 , 2 , 3 , 4 ]. The searches for new drug delivery systems approach and new modes of action represent one of the frontier research areas. Those involve multi-disciplinary scientific approaches to provide major advances in an improving therapeutic index and bioavailability at the specific delivery of drugs [ 5 , 6 ]. Drug delivery system combines one or more traditional drug delivery systems with engineered technologies. The systems create the ability to specifically targeting point where a drug has released in the body and/or the rate at which it has released.
Biodegradable and bio-absorbable polymers make the magic possible choice for lot of new drug delivery systems. The bio-absorbable polymers like hydrogels such as poly (lactic acid) and poly (glycolic acid), and their copolymers have used to create the delivery component of the systems [ 7 , 8 ]. Whether the drug delivery system relies on a biodegradable implant to deliver medicine subcutaneously or deep within the body, the biodegradable and bio-absorbable polymers provide a safe framework for delivering medicine without harm to the body.
Polymeric drug delivery system has defined as a formulation or a device that enables the introduction of a therapeutic substance into the body. It improves its safety and efficacy by controlling the rate, time, and place of release of drugs in the body. Drug delivery has achieved great development in the last two decades, but it remains a difficult task to regulate drug entry into the body such as brain. However, recent progress in studies of the carrier-mediated transportation of nano-drug delivery system across the blood-brain barrier is beginning to provide a rational basis for controlling drug distribution to the brain. The transport systems at the blood-brain barrier are the uptake transporters for natural nutrients such as amino acid, peptide, hexose, mono-carboxylate and stem cells [ 9 , 10 , 11 ].
The present paper has been reviewed for the polymeric drug and gene delivery systems of natural and synthetic polymers to formulate drugs into the backbone structures in various cases. The future prospects of the research for practical applications has been also proposed for the development in the fields.
Natural polymers for drug delivery
Arginine derivatives.
Arginine, also known as l -arginine, is α-amino acid that uses in the biosynthesis of proteins [ 12 ]. It contains α-amino group, α-carboxylic acid group, and a side chain consisting of a 3-carbon aliphatic straight chain ending in a guanidino group as shown in Fig. 1 . At physiological pH, the carboxylic acid is deprotonated (−COO − ), the amino group is protonated (−NH 3 + ), and the guanidino group is protonated to give the guanidinium form (−C-(NH 2 ) 2 + ), making arginine a charged aliphatic amino acid [ 13 ]. The amino acid side-chain of arginine consists of a 3-carbon aliphatic straight chain, the distal end of which is capped by a guanidinium group, which has a pK a of 12.48. It is therefore always protonated and positively charged at physiological pH. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized, enabling the formation of multiple hydrogen bonds in the chemical structures [ 14 ].
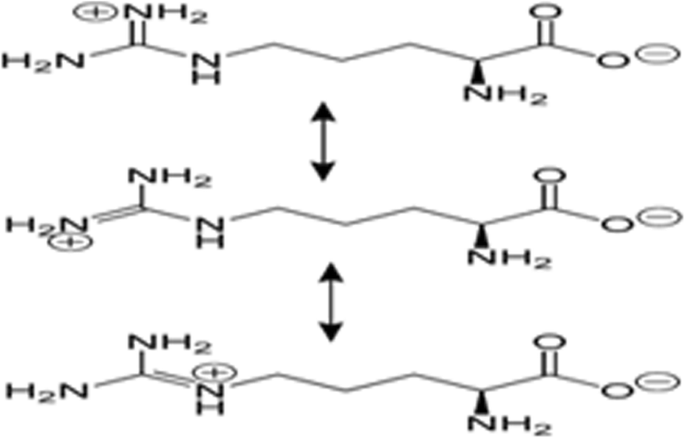
The delocalization of charge in guanidinium group of l -arginine for polymeric drug delivery systems
Chitosan derivatives
Chitosan is one of cationic polysaccharides derived from the natural chitin.
As a cationic polymer with favorable property, it has been widely used to form polyelectrolyte complexes with polyanions for drug delivery [ 15 , 16 ]. Chitosan is a linear copolymer composed by glucosamine and N -acteyl glucosamine units, via β-(1, 4) linkages, namely 2-amino-2-deoxy-β-d-glucan (Fig. 2 a). Chitosan is the product of the deacetylation reaction of chitin (2-acetamido-2-deoxy-β-d-glucan). It has favorable biological properties such as nontoxicity, muco-adhesiveness, biocompatibility and the biodegradability [ 17 , 18 , 19 ]. The aqueous derivatives of chitosan such as chitosan salts (Fig. 2 b), zwitterionic chitosan, and chitosan oligomers have drawn increasing attention due to their water-solubility for biomedical applications [ 20 , 21 , 22 , 23 ].
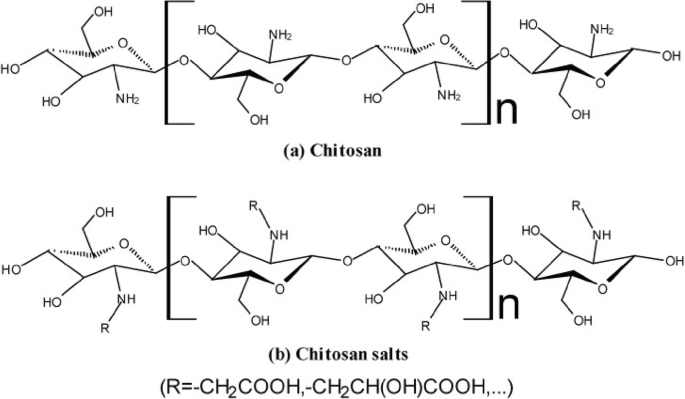
The chemical structures of chitosan ( a ) and chitosan salts ( b )
Cyclodextrin derivatives
Cyclodextrin is a family of cyclic oligosaccharides composed of α (1, 4) linked glucopyranose subunits. Cyclodextrin is useful molecular chelating agent. There are three types of cyclodextrins in the nature. Those are named α (6 units), β (7 units) and γ -cyclodextrins (8 units) as shown in Fig. 3 . β -Cyclodextrin is ideal for drug delivery due to the cavity size, efficiency drug complexation and loading, availability and relatively low cost [ 24 ]. An example of cyclodextrin in drug delivery system is 2-hydroxylpropyl derivate, which is a powerful solubilizer, and has a hydrophilic chain outside and a hydrophobic chain inside [ 25 ]. They are able to prevent the drug degradation and to improve the drug stability and solubility resulting on a higher bioavailability [ 26 , 27 ]. Those are very useful for polymeric drug delivery systems for practical applications.
The chemical structure of the three main types of cyclodextrin (CD) for polymeric drug delivery systems
Poly (glycolic acid), poly (lactic acid), and hyaluronic acid
Glycolic acid is a useful intermediate for organic synthesis, in a range of reactions, including oxidation-reduction, esterification, and long chain polymerization. It has used as a monomer in the preparation of polyglycolic acid and other biocompatible copolymers. Two molecules of lactic acid have dehydrated to the lactone lactide. In the presence of catalysts, lactides polymerize to either atactic or syndiotactic polylactide which are biodegradable polyesters [ 28 ]. Glycolic acid and lactic acid have employed in pharmaceutical technology to produce water-soluble glycolate and lactate from otherwise-insoluble active ingredients. They have found further to use in drug delivery, topical preparations, and cosmetics to adjust acidity and for its disinfectant and keratolytic properties [ 29 , 30 ]. Hyaluronic acid, which is a natural polymer, has the ability to target the CD44 over expressing cancer cells.
Polysaccharides
Natural polymers have been in use for many years with the aim of facilitating the efficiency of drugs and their delivery. Biodegradable polymers are widely being studied as a potential carrier material for specific drug delivery because of their non-toxic, biocompatible nature. Natural polysaccharides have investigated for application in drug delivery industry as well as in biomedical fields. Modified polymer has found its application as a support material for gene delivery, cell culture, and tissue engineering. Nowadays, natural polymers have modified to obtain novel biomaterials for controlled drug delivery applications.
Polysaccharides are long chains of carbohydrate molecules, specifically polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages as shown in Fig. 4 . This carbohydrate can react with water-hydrolysis using amylase enzymes at catalyst, which produces constituent sugars (monosaccharides or oligosaccharides). Natural saccharides are generally of simple carbohydrates called monosaccharides with general formula (CH 2 O) n where n is three or more. Examples of monosaccharides are glucose, fructose, and glyceraldehyde [ 31 ]. Those natural polymers have used as biomaterials for drug delivery systems. Starch is a glucose polymer in which glucopyranose units have bonded by alpha -linkages. It has made up of a mixture of amylose and amylopectin. Amylose consists of a linear chain of several hundred glucose molecules and amylopectin is a branched molecule made of several thousand glucose units [ 32 ].
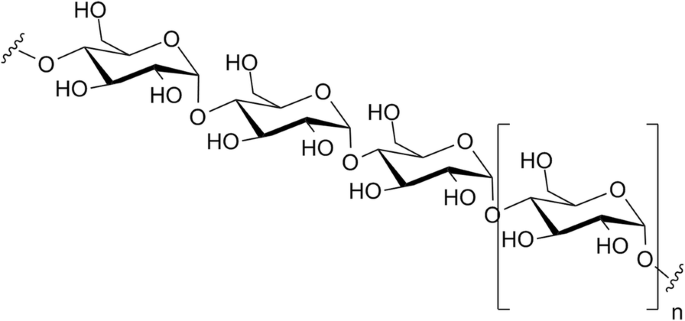
Amylose is a linear polymer of glucose mainly linked with α (1 → 4) bonds. It is one of the two components of starch polymer
Synthetic polymers for drug delivery systems
Poly(2-hydroxyethyl methacrylate).
Poly(2-hydroxyethyl methacrylate) [poly (HEMA)] is a polymer that forms a hydrogel in water or aqueous solution [ 33 ]. Poly (PHEMA) hydrogel for intraocular lens material was synthesized by solution polymerization using 2-hydroxyethyl methacrylate (HEMA) as raw material, azobisisobutyronitrile (AIBN), ammonium persulfate or sodium pyrosulfite (APS/SMBS) as catalyst, and ethyleneglycoldimethacrylate (EGDMA) or triethyleneglycoldimethacrylate (TEGDMA) as cross-linking additive [ 34 ]. Poly (HEMA) is commonly used to coat cell culture flasks in order to prevent cell adhesion and induce spheroid formation, particularly in cancer research. Older alternatives to pHEMA include agar and agarose gels [ 35 , 36 ]. Equilibrium swelling, structural characterization and solute transports in swollen poly (HEMA) gels cross-linked with tripropyleneglycol diacrylate (TPGDA) were investigated for a wide range of TPGDA concentrations for drug delivery systems [ 37 ]. The physical and chemical properties of pilocarpine from poly (HEMA) hydrogels were investigated to elucidate the mechanism of drug–polymer interaction and the effect on drug release behavior of controlled release polymeric devices [ 38 ]. Poly (HEMA) hydrogels are widely used for biomedical implants. The extreme hydrophilicity of poly (HEMA) confers resistance to protein fouling, making it a strong candidate coating for ventricular catheters [ 39 ].
Poly(N-isopropyl acrylamide)s
Aqueous solution of poly(N-isopropyl acrylamide) (PNIPAAm) shows a lower critical solution temperature (LCST). The temperature-responsive polymer has investigated in the 1960’s [ 40 ]. They have established 32 C as the LCST of thermos-sensitive poly(N-isopropyl arylamide). The thermodynamic property of the system has evaluated from the phase diagram and the heat absorbed during phase separation by entropy effect [ 41 ]. The process of free radical polymerization for a single type of monomer, in this case of N -isopropyl-acrylamide, find to form the polymer known as a homo-polymerization. The initiator of azobisisobutyronitrile (AIBN) has commonly used in radical polymerization.
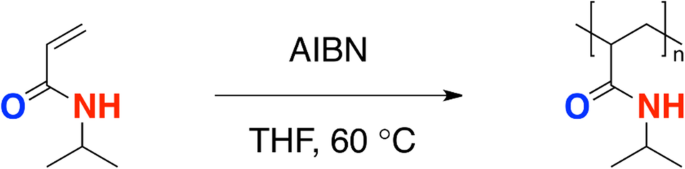
Thermo-responsive polymers have attracted much attention because of their potential biological and medical applications such as drug and gene delivery [ 42 , 43 , 44 ]. The swelling of cross-linked poly( N , N ′-alkyl substituted acrylamides) in water was studied in relation to temperature changes. The thermo-sensitivity of water swelling has attributed to the delicate hydrophilic/hydrophobic balance of polymer chains and has affected by the size, configuration, and mobility of alkyl side-chain groups [ 45 ].
The cell culture surface of the polymer has readily prepared by the technique reversibly into hydrophilic and hydrophobic coatings of PNIPAAm-grafted polymers [ 46 ]. Temperature/pH sensitive hydrogels were prepared by copolymerizing N-isopropyl acrylamide (NIPAAm) and acrylic acid (AAc) [ 47 ]. The influence of polyelectrolyte on the LCST of temperature/pH sensitive hydrogels had investigated in the pH range of swelling ratio. The swelling ratio of the hydrogels in the presence of poly (allyl amine) (PAA) as a polyelectrolyte was also measured at the same conditions [ 48 ]. It has briefly discussed about the tumor micro-environmental responsive nano-particles in situ stimuli responsive such as pH, redox responsive, hypoxia sensitive, etc.
Poly (ethylenimine)s
Linear poly (ethylenimine)(PEI) is soluble in hot water, at low pH, ethanol or chloroform. They are insoluble in cold water, acetone, benzene, and ethyl ether. Branched PEI has synthesized by the ring opening polymerization of aziridine as shown in Fig. 5 . Linear PEI is available by post-modification of other polymers like poly (2-oxazolines) or N -substituted polyaziridines [ 49 ]. Linear PEI was synthesized by the hydrolysis of poly (2-ethyl-2-oxazoline) [ 50 , 51 ].
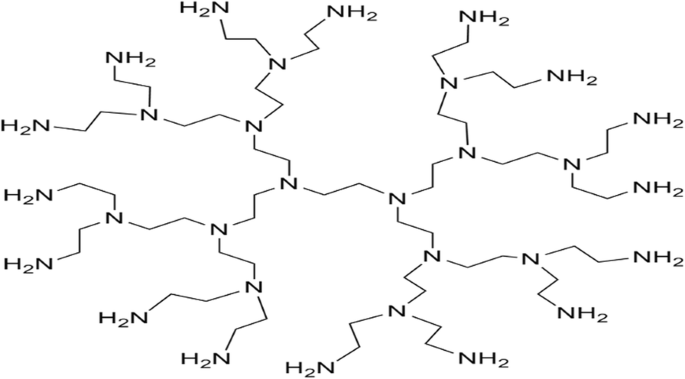
The chemical structure of poly (ethylenimine) s for polymeric drug delivery
Poly(N-(2-hydroxypropyl) methacrylamide)s
Degradable diblock and multiblock (tetrablock and hexablock) N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-gemcitabine (GEM) and -paclitaxel (PTX) conjugates had synthesized by reversible addition-fragmentation chain-transter (RAFT) copolymerization followed by click reaction for preclinical investigation [ 52 ]. Poly (HPMA) copolymer-cytarabine and GDC-0980 conjugates were synthesized. In vitro studies demonstrated that both conjugates had potent cytotoxicity and their combination showed strong synergy, suggesting a potential chemotherapeutic strategy [ 53 ]. Telechelic water-soluble HPMA copolymers and HPMA copolymer-doxorubicin (DOX) conjugates had synthesized by RAFT polymerization mediated by a new bi-functional chain transfer agent that contained an enzymatically degradable oligopeptide sequence [ 54 , 55 ].
Dendritic polymers
Dendritic polymers are highly branched polymers with controllable structures, which possess a large population of terminal functional groups, low solution or melt viscosity, and good solubility. Their size, degree of branching and functionality can be controlled and adjusted through the synthetic procedures. The research of dendrimer has increased on the design and synthesis of biocompatible dendrimer and its application to many areas of bioscience including drug delivery, immunology and the development of vaccines, antimicrobials and antivirals [ 56 , 57 ].
The dendrimers are the members of a versatile, new class of polymer architectures, dendritic polymers after traditional linear, cross-linked, and branched types as shown in Fig. 6 and Fig. 7 . The dendrimer type of bio-reducible polymer for efficient gene delivery had been also investigated [ 58 ].
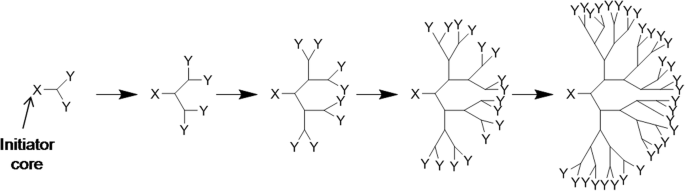
The schematic design of divergent synthesis of dendrimers for drug delivery
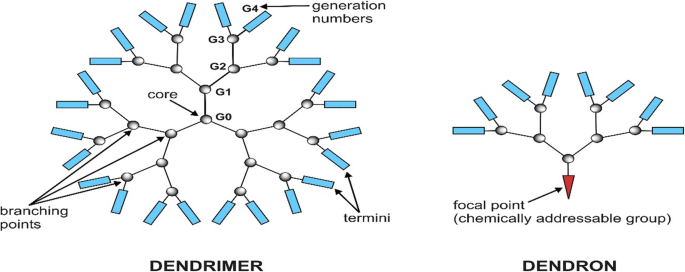
The chemical structures of dendrimer and dendron for drug delivery
Biodegradable and bio-absorbable polymers
Bio-absorbable drug delivery systems are a better choice for the application of drug carriers where only the temporary presence of the implant is needed [ 59 ]. Among the synthetic and biodegradable polymers, aliphatic polyesters such as poly (glycolic acid), poly (lactic acid), poly (caprolactone) and polydioxanone, are most commonly used and applied to drug delivery systems. As shown in Fig. 8 , the several classes of polymers such as poly (esters), poly (ortho esters), polyanhydrides, and biodegradable polycarbonates have also been introduced as potential implant materials for drug delivery [ 60 , 61 , 62 ].
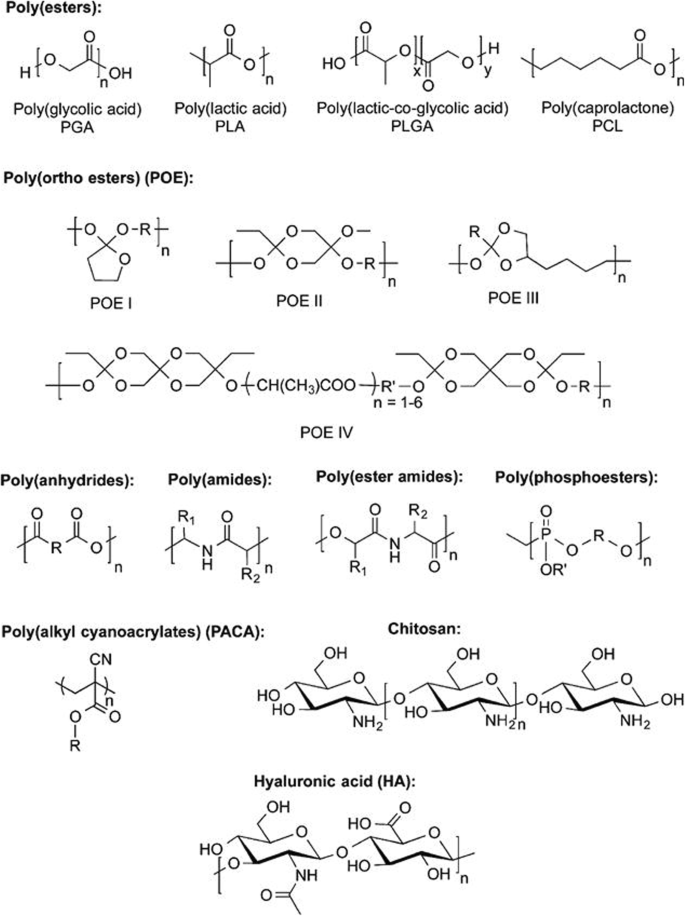
Biodegradable polymers with representative monomer units for polymeric drug delivery
Biodegradable polymers commonly used include the α -hydroxy acids, polyanhydrides, poly (amides), poly (ester amides), poly (phosphoesters), poly (alkyl cyanoacrylates), poly (hyarulonic acids) and natural sugars such as chitosan, in addition to many other types of degradable polymers as shown in Fig. 7 Synthetic biodegradable polymers are favored in drug delivery systems, as they have immunogenicity as compared to biodegradable polymers from natural polymers [ 63 , 64 , 65 ].
Polymeric drug delivery systems
Targeting polymeric drug delivery.
The therapeutic targeting of biomimetic chitosan-PEG-folate-complexed oncolytic adenovirus has examined for active and systematic cancer gene therapy [ 66 ]. The oncolytic adenovirus coated with multi-degradable bio-reducible core-cross-linked poly (ethyleneimine) for cancer gene therapy had been also applied [ 67 ]. Hepatoma targeting peptide conjugated bio-reducible polymer complexed with oncolytic adenovirus for cancer gene therapy were investigated [ 68 ]. Despite considerable advances in tumor-targeting technologies, the lack of selectivity towards tumor cells is still the primary limitation of current cancer therapies. A novel strategy for targeted drug delivery to cancer cells had developed through the formation of a physical conjugate between doxorubicin (Dox) and the A10 RNA aptamer that binds to the prostate-specific membrane antigen (PSMA) [ 69 ].
The effective polymers have designed specifically for gene delivery, and much has learned about their structure–function relationships. With the growing understanding of polymer gene-delivery mechanisms and continued efforts of creative polymer scientists, it is likely that polymer-based gene-delivery systems will become an important tool for human gene therapy [ 70 ].
Nanoparticle-based therapeutics in lung cancer is an emerging area and covers the diagnosis, screening, imaging, and treatment of primary and metastatic lung tumors. Innovative engineering on polymeric nano-carriers allows multiple anticancer drugs and gene delivery to site-specific targets [ 71 ]. The targeted drug delivery and gene therapy through natural biodegradable nanoparticles is an area of major interest in the field of nanotechnology and pharmaceuticals [ 72 ].
Biomimetic and bio-inspired polymers
The biomimetic and bioinspired systems improve biocompatibility during drug delivery application. The success of such a drug delivery system depends on parameters like shape, surface, texture, movement, and preparation methods. The systems have great influence on the biological systems owing to their less toxicity, high biocompatibility, significant interaction, and so on [ 73 , 74 , 75 ]. The novel developments of dendritic polymers based targeting nanoscale drug delivery vehicles described here provide great potential to achieve better therapeutic indexes in cancer therapy as well as low side effect [ 76 , 77 , 78 ]. Although synthetic drug carriers have developed for many applications, it remains important to examine natural particulates, which range from pathogens to mammalian cell’s mechanisms. Biocompatible polymeric nanoparticles are considerably promising carrier candidates in delivery of drugs and genes because of their unique chemical and physical properties [ 79 , 80 ].
Drug-free macromolecular therapeutics
Drug-free macromolecular therapeutics induce apoptosis of malignant cells by the crosslinking of surface non-internalizing receptors. The receptor crosslinking has mediated by the bio-recognition of high-fidelity natural binding motifs. Those have grafted to the side chains of polymers or attached to targeting moieties against cell receptors. This approach features the absence of low-molecular-weight cytotoxic compounds. Macromolecular therapeutics, also referred to as polymeric nano-medicines, are a diverse group of drugs characterized by their large molecular weight (MW), including polymer-drug conjugates, polymeric micelles, and polymer-modified liposomes [ 81 , 82 , 83 ].
Polymeric gene delivery systems
Gene therapy is a promising new technique for treating cancer and.
genetic disorders by introducing foreign genomic materials into host cells to elicit a therapeutic benefit. The gene therapy has a potential in treating many diseases such as infectious disease and immune system disorders. The efficient delivery of therapeutic gene to target a cell is the most important step in gene therapy [ 84 , 85 ]. Successful gene therapy is thus dependent on the development of an efficient delivery vector. There are non-viral vectors and viral vectors for gene delivery [ 86 ]. Pulmonary drug and gene delivery to the lung represents a non-invasive avenue for local and systemic therapies. Nano-sized particles offer novel concepts for the development of optimized therapeutic tools in pulmonary research. Polymeric nano-carriers are generally preferred as controlled pulmonary delivery systems due to prolonged retention in the lung [ 87 ].
Non-viral vectors for gene delivery
Polyethylenimine derivatives.
Polyethylenimine (PEI) is a class of cationic polymers proven to effect for gene delivery [ 88 ]. Branched poly (ethylenimine)(PEI) 25 kDa is an efficient gene delivery vector with outstanding gene condensation ability and great endosome escape activity [ 89 ]. A bio-reducible polyethylene-imine (PEI (−s-s-)) was derived from low molecular weight PEI (1.8 kDa) for efficient gene delivery. The bio-reducible core molecules have expected to increase molecular weights and reduce the cytotoxicity of the copolymers. PEI (−s-s-) polyplexes showed higher transfection efficiency and lower cytotoxicity compared to branched PEI 25 kDa, Lipofectamine® 2000. In addition, PEI (−s-s-) derivatives (16 kDa) had formed stable polyplexes with a zeta-potential value of + 34 mV and the size of polyplex 61 nm [ 90 ]. The cytotoxicity of polyethylenimine (PEI) is a dominating obstacle to its application. Polyethylenimine (PEI) is a well-known cationic polymer, which has high transfection efficiency owing to its buffering capacity. It has reported that PEI is cytotoxic in many cell lines and non-degradable. In order to solve the problems, the polyethylenimine copolymers have introduced firstly in gene delivery systems [ 91 ].
Polyethylenimine copolymers
The introduction of poly (ethylene glycol) (PEG) blocks to PEI is one of the.
strategies to alleviate the cytotoxocity of PEI. However, it has well known that the transfection efficiency of PEGylated PEI has decreased to some extent compared to the corresponding PEI. Novel ABA triblock copolymers consisting of low molecular weight linear polyethylenimine (PEI) as the A block and poly (ethylene glycol) (PEG) as the B block were prepared and evaluated as polymeric transfectant. The PEI-PEG-PEI triblock copolymers displayed also an improved safety profile in comparison with high molecular weight PEIs. The linear PEI-PEG-PEI triblock copolymers are an attractive novel class of non-viral gene delivery systems [ 92 ].
Polyethylenimine- alt -poly (ethylene glycol) copolymers had been synthesized for an ideal gene carrier both safety and transfection efficiency. The copolymers were complexed with plasmid DNA. The resulting complexes exhibited no cytotoxic effects on cells even at high copolymer concentration. It’s transfection efficiency was influenced by poly (ethylene glycol)(PEG) molecular weight. The transfection efficiency was higher than that for PEI 25 K in HepG2 and MG63, whereas it was lower than that for PEI 25 K in HeLa cells [ 93 ].
Aiming to prepare a biodegradable gene vector with high transfection efficiency and low cytotoxicity, it had conjugated low molecular weight (LMW) PEIs to the biodegradable backbone polyglutamic acids derivative (PEG-b-PBLG) by aminolysis to form PEIs combined PEG- b -PLG- g -PEIs [ 94 ]. A series of tri-block co-polymers, PEG- g -PEI- g -poly (dimethylaminoethyl L-glutamine) (PEG- g -PEI- g -PDMAEG), as novel vectors for gene therapy was synthesized and evaluated [ 95 ]. The synthesized PEG-g-PEI-g-PDMAEG tri-block copolymers are promising candidates as non-viral carriers for gene delivery.
Polyethylenimine conjugated bio-reducible polymers
In order to introduce the disulfide bond between poly (cystamine- bis - (acrylamide) diaminohexane) [poly (CBA-DAH)] and PEI 1.8 kDa, Traut’s reagent were used to synthesize the products [ 96 ]. Poly (CBA-DAH)-PEI can be confirmed its potential as a gene delivery carrier. For the identification of the products, the proton peaks of poly (CBA-DAH) and PEI were shifted downfield due to steric hindrance caused by the conjugation between P (CBA-DAH) and PEI. In addition, the conjugation ratio of PEI to the PCDP has been calculated by the ratio of the integration of the proton spectra peaks in poly (CBA-DAH)(−NCH 2 CH 2 CH 2 CH 2 -CH 2 CH 2 NH 2 ) and CH 2 of PEI. Poly (ethylenimine) (PEI, 1.8 kDa) was conjugated to poly (CBA-DAH) via a disulfide bond. The PEI conjugated poly (CBA-DAH)[PCDP] was able to bind with pDNA at a very low molecular weight ratio and form the polyplexes with nano-size and positive surface charge.
The PCDP polyplexes show 10 times higher gene transfection efficiency than Lipofectamine® polyplexes in bio-mimic in vivo condition. The bio-reducible PEI (1.8 kDa) conjugated poly (CBA-DAH) is finally concluded as an efficient polymeric gene delivery carrier [ 97 , 98 ]. It has been concluded that the PEI(1.8 kDa)-PCDP synthesized in our laboratory is one of the good candidates as mRNA, siRNA, and pDNA carriers for efficient gene delivery systems [ 99 ]. Outstanding representatives of bio-polymers that have emerged over the last decade to be used in gene therapy are synthetic bio-reducible polymers such as poly( l -lysine), poly( l -ornithine), linear and branched polyethyleneimine, diethyl-aminoethyl-dextran, poly (amidoamine) dendrimers, and poly (dimethyl-aminoethyl methacrylate) [ 100 ].
Viral vectors for polymeric gene delivery
Viral vectors not only have the ability to effectively infect cells, but also transfer DNA to the host without causing an immune response. Viral vectors have designed to be safe by making them incapable of replication. Gene transferred by viral vector has dominated the clinical trials in gene therapy, because they are more efficient than physicochemical methods [ 101 ]. Viral vectors have divided into two types, which are integrating and non-integrating viral vectors. Integrated viral vectors have integrated into the human genome, including adeno-associated virus and retroviral vectors; non-integrating vectors, like adenoviral vectors. They remain in the nucleus without having integrated into the chromosomal DNA and RNA. Gene delivery systems for gene therapy provide a great opportunity for treating diseases from genetic disorders, cancer, and other infections. The recent development of gene delivery system has reviewed for viral delivery systems and non-viral delivery systems [ 102 ].
DNA conjugates
Gene therapy is a promising new technique for treating many serious incurable diseases such as cancer and genetic disorders. The main problem limiting the application of this strategy in vivo is the difficulty of transporting large, fragile and negatively charged molecules like DNA into the nucleus of the cell without degradation [ 103 ]. The gene therapy of DNA conjugate is as a new promising technique used to treat many incurable diseases and the different strategies used to transfer DNA, taking into account that introducing DNA into the cell nucleus without degradation. It is essential for the success of this therapeutic technique.
The use of DNA as a drug is both appealing and simple in concept. In many instances, the feasibility of such an approach has been established using model systems. In practical terms, the delivery of DNA to human tissues presents a wide variety of problems that differ with each potential therapeutic application [ 104 ]. The challenge for the therapeutic use of viral vectors is to achieve efficient and often extended expression of the exogenous gene while evading the host defenses. Recent engineering of modified viral vectors has contributed to improved gene delivery efficacy [ 105 ]. The design of polymeric nanoparticles for gene therapy requires engineering of polymer structure to overcome multiple barriers, including prolonged colloidal stability during formulation and application. Poly(β-amino ester) s have been shown effective as polymeric vectors for intracellular DNA delivery [ 106 ].
RNA conjugates
Most of the current methods for programmable RNA drug therapies are unsuitable for the clinic due to low uptake efficiency and high cytotoxicity. RNA therapeutics including small-interfering RNAs (siRNAs), antisense oligonucleotides (ASOs), and CRISPR–Cas9 genome editing guide RNAs (gRNAs) are emerging modalities for programmable therapies that target the diseased human genome with high specificity and great flexibility [ 107 ]. RNA interference (RNAi) mediated gene silencing holds significant promises in gene therapy. A major obstacle to efficient RNAi is the systemic delivery of the therapeutic RNAs into the cyto-plasma without having trapped in intracellular endo-lysosomes [ 108 ].
RNA interference (RNAi) has been proven to be an useful approach to treat various genetic diseases. It can down-regulate specific protein expression by silencing the activity of its targeted gene [ 109 , 110 ]. RDG could tightly condense shRNAs into stable complex nanoparticles. The RDG/shRNA nanoparticle had found to be highly selective in targeting the U-87 MG-GFP cells with over-expressed αvβ3 integrins via receptor-mediated endocytosis. The RDG/shRNA complex, which combines RGD-mediated active targeting and glutathione-triggered intracellular release and low cytotoxicity, appears to be a highly promising non-viral vector for efficient RNA delivery and therapy [ 111 , 112 ]. Exosomes, unlike other vectors for gene delivery, present unique advantages such that exosomes are a cell-free natural system for ferrying RNA between cells, robust exosomal membrane can protect the RNA/gene of interest from digestion, and exosomes are rapidly taken up by target cells making them a more efficient vehicle for gene delivery [ 113 ]. Delivery of these miRNA molecule enriched-exosomes subsequently results in highly efficient overexpression or deletion of the designated miRNAs in the recipient cells both in vivo and in vitro [ 114 ].
Conclusion and future prospects
The development of drug delivery carriers based on natural and synthetic polymers are rapidly emerging field. It takes advantages of the remarkable delivery mechanism, which has used by pathogens and mammalian cells, such as selective targeting and prolonged circulation by evasion of the immune systems. The biomimetic and bio-inspired systems have a bright future ahead with a lot of potentials to solve any obstacles encountered in polymeric drug delivery. The fruitful progress will have made in the application of biocompatible and bio-related copolymers and dendrimers to cancer treatment, including their use as delivery systems for potent anti-cancer drugs such as cis -platin and doxorubicin. The unique properties of dendrimers such as their high degree of branching, multi-valence, globular architecture, and well-defined molecular weight make them promising new scaffolds for polymeric drug delivery systems.
The micro-processes that are required for the development of such carriers, such as genetic engineering or in vivo treatments to incorporate therapeutic substances, make it difficult to maintain the integrity of natural and synthetic polymers with cells in a body. The gap between synthetic and biological systems has traditionally been very large. Recent advances in the synthesis of novel biomaterials and understanding of biological systems have paved the way towards bridging this gap. Polymeric drug delivery carriers that have based on pathogens such as bacteria and viruses are potentially immunogenicity for human body. A certain degree of immunogenicity can be ideal if pathogen-based carriers have intended for vaccine delivery, owing to their adjuvant ability. Combining perspectives from the synthetic and biological fields will provide a new paradigm for the design of polymeric drug delivery systems in near future.
Availability of data and materials
Not applicable.
Abbreviations
Acrylic acid
Azobisisobuyronitrile
Ammonium persulfate or sodium pyrosulfite
Cyclodextrin
Ethyleneglycol dimethacrylate
Doxorubicin
Gemcitabine
Hydroxyethyl methacrylate
N-(2-hydroxypropyl) methacrylamide
Lower critical solution temperature
Low molecular weight
Molecular weight
N-isopropyl acrylamide
Poly (allyl amine)
Poly (CBA-DAH)
Poly (dimethylaminoethyl L-glutamine)
Poly (ethylene glycol- block - poly(L-glutamate)-graft-poly (ethylenimine)s
Poly (ethylene glycol- graft -poly (ethylenimine)- graft -poly (dimethyl amino ethyl L-glutamate)
Poly (ethylenimine)
Poly (ethylene glycol)
Poly(N-isopropyl acrylamide)
Poly (cystamine- bis -(acrylamide) diaminohexane
Poly (2- hydroxyethyl methacrylate)
Poly(N-(2- hydroxypropyl) methacrylamide)
prostate-Specific membrane antigen
Triethyleneglycol dimethacrylate
Tripropylene glycol diacrylate
Anderson JM, Kim SW. Advances in Drug Delivery Systems (3), Book Review. J Pharm Sci. 1989;78:608–9 [ Google Scholar ].
Article Google Scholar
Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AICHE J. 2003;49:2990–3006 [ Google Scholar ] .
Article CAS Google Scholar
Heller A. Integrated medical feedback systems for drug delivery. AICHE J. 2005;51:1054–66 [ Google Scholar ].
Martinho N, Damgé C, Pinto C. Reis, Recent advances in drug delivery systems. J Biomater Nanobiotechn. 2011;2:510–26 [ Google Scholar ].
Din F, Aman W, Ullah I, Quereshi OS, Mustapha O, Shafique S, Zeb A. Effective use of nano-carriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. 2017;12:7291–309 [ Google Scholar ].
Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2:2–11 [ Google Scholar ].
SinhaVR LK. Bio-absorbable polymers for implantable therapeutic systems, Drug Dev. Ind Pharm. 1998;24:1129–38 [ Google Scholar ].
Basua A, Kundurua KR, Doppalapudib S, Domba AJ, Khanb W. Poly (lactic acid) based hydrogels. Adv Drug Deliv Rev. 2016;107:192–205. https://doi.org/10.1016/j.addr.2016.07.004 [Google Scholar].
Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10:298–305. https://doi.org/10.3390/pharmaceutics10040269 [ Google Scholar ].
Cacciatore I, Ciulla M, Fornasari E, Marinelli L, Di Stefano A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases, Expert. Opin Drug Deliv. 2016;13(8):1121–31. https://doi.org/10.1080/17425247.2016 . [ Google Scholar ].
Patel M, Souto EB, Singh KK. Advances in brain drug targeting and delivery: limitations and challenges of solid lipid nanoparticles, Expert. Opin Drug Deliv. 2013;10:889–905 [ Google Scholar ].
Tapiero H, Mathé G, Couvreur P, Tew KD. L-arginine. Biomed Pharmacother. 2002;56:439–45. https://doi.org/10.1016/s0753-3322(02)00284-6 PMID 12481980 . [ Google Scholar ].
Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem. 2004;15:442–51 [ Google Scholar ].
Skipper A. Dietitian's Handbook of enteral and parenteral nutrition : Jones & Bartlett Learning; 1998. [ Google Scholar ].
Wu QX, Lin DQ, Yao SJ. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Marine Drugs. 2014;12:6236–53 [ Google Scholar ].
Hamman JH. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Marine Drugs. 2010;8:1305–22 [ Google Scholar ].
Onishi H, Machida Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials. 1999;20:175–82 [ Google Scholar ].
Xia WS, Liu P, Liu J. Advance in chitosan hydrolysis by non-specific celluloses. Bioresour Technol. 2008;99:6751–62 [Google Scholar].
Zhang H, Alsarra IA, Neau SH. An in vitro evaluation of a chitosan-containing multi-particulate system for macromolecule delivery to the colon. Int J Pharm. 2002;239:197–205 [ Google Scholar ].
Xu PS, Bajaj G, Shugg T, van Alstine WG, Yeo Y. Zwitterionic chitosan derivatives for pH-sensitive stealth coating. Biomacromolecules. 2010;11:2352–8 [ Google Scholar ].
Bajaj G, van Alstine WG, Yeo Y. Zwitterionic chitosan derivative, a new biocompatible pharmaceutical excipient, prevents endotoxin-mediated cytokine release. PLoS One. 2012;7:1–10 [ Google Scholar ].
Google Scholar
Čalija B, Cekić N, Savić S, Daniels R, Marković B, Milić J. pH-sensitive micro-particles for oral drug delivery based on alginate/oligo-chitosan/Eudragit®L100–55 “sandwich” polyelectrolyte complex. Colloid Surf B. 2013;110:395–402 [ Google Scholar ].
Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromol. 2014;64:353–67 [ Google Scholar ].
Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals, Biochim. Biophys Acta. 2009;1788:2362–73. https://doi.org/10.1016/j.bbamem.2009.08.015 [ Google Scholar ].
Manosroi J, Apriyani MG, Foe K, Manosroi A. Enhancement of the release of azelaic acid through the synthetic membranes by inclusion complex formation with hydroxypropyl-beta-cyclodextrin. Int J Pharm. 2005;293:235–40. https://doi.org/10.1016/j.ijpharm.2005.01.009 [ Google Scholar ].
Wang S, Tan M, Zhong Z, Chen M, Wang Y. Nanotechnologies for curcumin: An ancient puzzler meets modern solutions. J Nanomater. 2011;2011:1–8. https://doi.org/10.1155/2011/723178 [ Google Scholar ].
Martin EM, Valle D. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46. https://doi.org/10.1016/S0032-9592(03)00258-9 [ Google Scholar ].
Odile DC, Blanca MV, Didier B. Controlled ring-opening polymerization of lactide and glycolide. Chem Rev. 2004;104:6147–76 [ Google Scholar ].
Yoo DK, Kim D, Lee DS. Synthesis of Lactide from Oligomeric PLA: Effects of Temperature, Pressure, and Catalyst. Macromol Res. 2006;14:510–6 [ Google Scholar ].
Pourasghar M, Koenneke A, Meiers P, Schneider M. Development of a fast and precise method for simultaneous quantification of the PLGA monomers lactic and glycolic acid by HPLC. J Pharm Anal. 2019;9:100–7. https://doi.org/10.1016/j.jpha.2019.01.004 [ Google Scholar ].
Matthews CE, Van Holde KE, Ahern KG. Biochemistry. 3rd ed. NY: Benjamin Cummings; 1999. ISBN 0-8053-3066-6 . [ Google Scholar ].
Green MM, Blankenhorn G, Hart H. Which starch fraction is water-soluble, amylose or amylopectin. J Chem Educ. 1975;52:729–38. https://doi.org/10.1021/ed052p729 [ Google Scholar ].
Wichterle O, Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–8. https://doi.org/10.1038/185117a0 [ Google Scholar ].
Sung YK, Jhon MS, Gregonis DE, Andrade JD. Thermal and pulse nuclear magnetic resonance analysis of water in poly (2-hydroxyethyl methacrylate). J Appl Polym Sci. 1981;26:3719–26. Google Scholar .
Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front Bioeng Biotechnol. 2016;4:12–8. https://doi.org/10.3389/fbioe.2016.00012 PMCID: PMC4751256. PMID: 26904541 . [ Google Scholar ].
Friedrich JS, Claudia ER, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–24. https://doi.org/10.1038/nprot.2008.226 PMID 19214182 . [ Google Scholar ].
Ferreira L, Vidal MM, Gil MH. Evaluation of poly (2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int J Pharm. 2000;194:169–80. https://doi.org/10.1016/S0378-5173(99)00375-0 [ Google Scholar ].
Hsiue GH, Cheng CC. Poly(2-hydroxyethyl methacrylate) film as a drug delivery system for pilocarpine. Biomaterials. 2001;22:1763–9. https://doi.org/10.1016/S0142-9612(00)00336-7 [ Google Scholar ].
Hanak BW, Hsieh CY, Donaldson W, Browd SR, Lau KS, Shain W. Reduced cell attachment to poly (2-hydroxyethyl methacrylate)-coated ventricular catheters in vitro . J Biomed Mater Res B Appl Biomater. 2018;106:1268–79. https://doi.org/10.1002/jbm.b.33915 [ Google Scholar ].
Heskins M, Guillet JE. Solution properties of poly(N-isopropyl acrylamide). J Macromol Sci Part A Chem. 1968;2:1441–55 [ Google Scholar ].
Zheng L, Qiulin L, Duanguang Y, Yong G, Xujun L. Well-defined poly( N -isopropylacrylamide) with a bifunctional end-group: synthesis, characterization, and thermoresponsive properties, Designed. Monomers Polymers. 2013;16:465–74. https://doi.org/10.1080/15685551.2012.747165 [ Google Scholar ].
Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–70 [ Google Scholar ].
Ma Y, Hou S, Ji B, Yao Y, Feng X. A novel temperature-responsive polymer as a gene vector. Macromol Biosci. 2010;10:202–10 [ Google Scholar ].
Weber C, Richard H, Schubert US. Temperature responsive bio-compatible polymers based on poly (ethylene oxide) and poly (2-oxazoline)s. Prog Polym Sci. 2012;37:686–714 [ Google Scholar ].
Bae YH, Okano T, Kim SW. Temperature dependence of swelling of crosslinked poly(N,N′-alkyl substituted acrylamides) in water. J Polym Sci Part B Polym Phys. 1990;28:923–36. https://doi.org/10.1002/polb.1990.090280609 [ Google Scholar ].
Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropyl acrylamide). J Biomed Mater Res. 1993;27:1243–51. https://doi.org/10.1002/jbm.820271005 [ Google Scholar ].
Yoo MK, Sung YK, Lee YM, Cho CS. Effect of polyelectrolyte on the lower critical solution temperature of poly(N-isopropyl acrylamide) in the poly (NIPAAm- co -acrylic acid) hydrogel. Polymer. 2000;41:5713–9. https://doi.org/10.1016/S0032-3861(99)00779 [ Google Scholar ].
Zhang Q, Ko NR, Oh IK. Recent advances in stimuli-responsive degradable block copolymer micelles: synthesis and controlled drug delivery applications. Chem Commun. 2012;48:7542–52. https://doi.org/10.1039/c2cc32408c [ Google Scholar ].
Tanaka R, Ueoka I, Takaki Y, Kataoka K, Saito S. High molecular weight linear polyethylenimine and poly(N-methylethylenimine). Macromolecules. 1983;16:849–53. https://doi.org/10.1021/ma00240a003 [ Google Scholar ].
Weyts KF, Goethals EJ. New synthesis of linear polyethyleneimine. Polym Bull. 1988;19:13–9. https://doi.org/10.1007/bf00255018 [ Google Scholar ].
Brissault B, Kichler A, Guis C, Leborgne C, Danos O, Cheradame H. Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconjug Chem. 2003;14:81–587. https://doi.org/10.1021/bc0200529 [ Google Scholar ].
Yang J, Zhang R, Pan H, Li Y, Fang Y, Zhang L, Kopeček J. Backbone degradable N-(2-hydroxypropyl) methacrylamide copolymer conjugates with gemcitabine and paclitaxel: Impact of molecular weight on activity toward human ovarian carcinoma xenografts. Mol Pharm. 2017;14:1384–94. https://doi.org/10.1021/acs.molpharmaceut.6b01005 [ Google Scholar ].
Zhang R, Yang J, Zhou Y, Shami PJ, Kopeček J. N-(2-hydroxypropyl) methacrylamide copolymer–drug conjugates for combination chemotherapy of acute myeloid leukemia. Macromol Biosci. 2016;16:121–8 [ Google Scholar ].
Pan H, Yang J, Kopeckova P, Kopecek J. Backbone degradable multiblock N -(2-hydroxypropyl) methacrylamide copolymer conjugates via reversible addition−fragmentation chain transfer polymerization and thiolene coupling reaction. Biomacromolecules. 2011;12:247–52. https://doi.org/10.1021/bm101254e [ Google Scholar ].
Zhang L, Zhang R, Yang J, Wang J, Kopecek J. Indium-based and iodine-based labeling of HPMA copolymer-epirubicin conjugates: Impact of structure on the in vivo fate. J Control Release. 2016;240:306–18 [ Google Scholar ].
GilliesJean ER, Fréchet MJ. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43 [ Google Scholar ].
Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nano-medical applications. Drug Discov Today. 2010;15:171–85 [ Google Scholar ].
Nam HY, Nam K, Lee M, Kim SW, Bull DA. Dendrimer type bio-reducible polymer for efficient gene delivery. J Control Release. 2012;160:592–600. https://doi.org/10.1016/j.jconrel.2012.04.025 [ Google Scholar ].
Törmälä P, Pohjonen T, Rokkanen P. Bio-absorbable polymers: materials technology and surgical applications. Proc Inst Mech Eng. 1998;212:101–11. https://doi.org/10.1243/0954411981533872 [ Google Scholar ].
Pulapura S, Kohn J. Trends in the development of bio-resorbable polymers for medical applications. J Biomater Appl. 1992;6:216–50 [ Google Scholar ].
Lee TS, Bee ST. Polylactic Acid (second edition), A practical guide for the processing, manufacturing and applications of PLA, plastics design library: Elsevier; 2019. p. 53–95. Elsevier B.V. [ Google Scholar ].
Heller J, Barr J, YNg S, Abdellauoi KS, Gurny R. Poly (ortho esters): synthesis, characterization, properties and uses. Adv Drug Deliv Rev. 2002;54:1015–39. https://doi.org/10.1016/S0169-409X(02)00055-8 [ Google Scholar ].
Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Adv Drug Deliv Rev. 2002;54:889–910. https://doi.org/10.1016/S0169-409X(02)00050-9 [ Google Scholar ].
Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem Rev. 2016;116:2602–63 [ Google Scholar ].
Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–235. https://doi.org/10.1039/c2cs35265f [ Google Scholar ].
Kwon OJ, Kang E, Choi JW, Kim SW, Yun CO. Therapeutic targeting of chitosan-PEG-folate-complexed oncololytic adenovirus for active and systematic cancer gene therapy. J Control Release. 2013;169:257–65 [ Google Scholar ].
Choi JW, Nam JP, Nam K, Lee YS, Yun CO, Kim SW. Oncolitic adenovirus coated with multi-degradable bio-reducible core-cross-linked poly-(ethyleneimine) for cancer gene therapy. Biomacromolecules. 2015;16:592–600. https://doi.org/10.1021/acs.biomac.5b00538 [ Google Scholar ].
Choi JW, Kim HA, Nam K, Na Y, Yun CO, Kim SW. Hepatoma targeting peptide conjugated bioreducible polymer complexed with oncolytic adenovirus for cancer gene therapy. J Control Release. 2015;220:691–703. https://doi.org/10.1016/j.jconrel.2015.09.068 [ Google Scholar ].
Pack DW, Hoffman A, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–93 [ Google Scholar ].
Ray L, Polymeric nanoparticle-based drug/gene delivery for lung cancer, in nanotechnology-based targeted drug delivery systems for lung cancer, 2019; Chap. 4: 77–93 [ Google Scholar ].
Pandey VN, Tiwari N, Pandey VS, Rao A, Das I. Targeted drug delivery and gene therapy through natural biodegradable nanostructures in pharmaceuticals, in nanoarchitectonics in biomedicine, vol. 13; 2019. p. 437–72. Chap. [ Google Scholar ].
Gu Z. Bioinspired and biomimetic polymer systems for drug and gene delivery: Chemical Industry Press and Wiley-VCH Verlag GmbH & Co.; Wiley. KGaA; 2015. ISBN: 9783527334209 |Online ISBN: 9783527672752. [ Google Scholar ].
Bagalkot V, Farokhzad OC, Langer R, Jon S. An aptamer–doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew Chem Int Ed. 2006;45:8149–52 [ Google Scholar ].
Speck O, Speck D, Horn R, Gantner J, Sedlbauer KP. Biomimetic bio-inspired biomorph sustainable. An attempt to classify and clarify biology-derived technical developments. Bioinspir Biomim. 2017;12:11004–6. https://doi.org/10.1088/1748-3190/12/1/011004 [ Google Scholar ].
Vincent JF. Biomimetics: a review. Proc Inst Mech Eng H. 2009;223:919–39. https://doi.org/10.1243/09544119JEIM561 [ Google Scholar ].
Sabua C, Rejob C, Kottab S, Pramoda K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J Control Release. 2018;287:142–55. https://doi.org/10.1016/j.jconrel.2018.08.033 [ Google Scholar ] .
Safari J, Zarnegar Z. Advanced drug delivery systems: Nanotechnology of health design A review. J Saudi Chem Soc. 2014;18:85–99 [ Google Scholar ].
Li Y, Thambi T, Lee DS. Co-delivery of drugs and genes using polymeric nanoparticles for synergistic cancer therapeutic effects. Adv Healthcare Mater. 2018;7:1700886. https://doi.org/10.1002/adhm.201700886 [ Google Scholar ].
Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nature Rev Drug Disc. 2011;10:521–35. https://doi.org/10.1038/nrd3499 [ Google Scholar ].
Yang L, Li J, Kopeček J. Biorecognition: A key to drug-free macromolecular therapeutics. Biomaterials. 2019;190:11–23 [ Google Scholar ].
Li J, Yang S, Soodvilai J, Wang P, Opanasopit J, Kopeček J. Drug-free albumin triggered sensitization of cancer cells to anticancer drugs. J Control Release. 2019;293:84–93 [ Google Scholar ].
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71–82. https://doi.org/10.1186/s12951-018-0392-8 [ Google Scholar ].
Li J, Yang J, Wang J, Kopeček J. Drug-free macromolecular therapeutics exhibit amplified apoptosis in G2/M phase arrested cells. J Drug Target. 2018;27:566–72 [ Google Scholar ].
Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature. 1997;389:239–42 [ PubMed ] [ Google Scholar ].
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–55 [ PubMed ] [ Google Scholar ].
Beck-Broichsitter M, Merkel OM, Kissel T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J Control Release. 2012;161:214–24. https://doi.org/10.1016/j.jconrel.2011.12.004 [ Google Scholar ].
Guo X, Huang L. Recent advances in non-viral vectors for gene delivery. Acc Chem Res. 2012;45:971–9. https://doi.org/10.1021/ar200151m [ Google Scholar ].
Nam K, Jung S, Nam JP, Kim SW. Poly (ethylenimine) conjugated bio-reducible dendrimer for efficient gene delivery. J Control Release. 2015;220:447–55. https://doi.org/10.1016/j.jconrel.2015.11.005 [Google Scholar].
Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23:8–12. https://doi.org/10.1186/s40824-019-0156-z [Google Scholar].
Zhong Z, Feijen J, Lok MC, Hennink WE, Christensen LV, Yockman JW, Kim YH, Kim SW. Low molecular weight linear polyethylenimine- b -poly (ethylene glycol)- b -polyethylenimine triblock copolymers: synthesis, characterization, and in vitro gene transfer properties. Biomacromolecules. 2005;6:3440–8 [Google Scholar].
Park MR, Han KO, Han IK, Cho MH, Nah JW, Choi YJ, Cho CS. Degradable polyethylenimine- alt -poly (ethylene glycol) copolymers as novel gene carriers. J Control Release. 2005;105:367–80 [Google Scholar].
Wen Y, Pan S, Luo X, Zhang X, Zhang W, Feng M. A biodegradable low molecular weight polyethylenimine derivative as low toxicity and efficient gene vector. Bioconjug Chem. 2009;20:322–32. https://doi.org/10.1021/bc800428y [ Google Scholar ].
Wen Y, Pan S, Luo X, Zhang W, Shen Y, Feng M. PEG- and PDMAEG-graft-modified branched PEI as novel gene vector: synthesis, characterization and gene transfection. J Biomater Sci Polym Ed. 2010;21:1103–26 [ Google Scholar ].
Kim SW, Nam JP, Kim S, Sung YK. Recent development of bio-reducible polymers for efficient gene delivery system. J Cancer Treatment Diagn. 2018;2:17–23 [ Google Scholar ].
Nam JP, Park JK, Son DH, Kim TH, Park SJ, Park SC, Choi C, Jang MK, Nah JW. Evaluation of polyethylene glycol conjugated novel polymeric antitumor drug for cancer therapy. Colloids Surf B: Biointerfaces. 2014;120:168–75 [ Google Scholar ].
Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel Biodegradable Poly (disulfide amine) s for Gene Delivery with High Efficiency and Low Cytotoxicity. Bioconjug Chem. 2008;19:626–33 PMID: 18314939. [Google Scholar].
Nam JP, Kim S, Kim SW. Design of PEI-conjugated bio-reducible polymer for efficient gene delivery. Int J Pharm. 2018;545:295–305. https://doi.org/10.1016/j.ijpharm.2018.04.051 [ Google Scholar ].
Lee YS, Kim SW. Bioreducible polymers for therapeutic gene delivery. J Control Release. 2014;190:424–39. https://doi.org/10.1016/j.jconrel.2014.04.012 [Google Scholar].
Rai R, Alwani S, Badea I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers (Basel). 2019;11:745–9. https://doi.org/10.3390/polym11040745 [ Google Scholar ].
Kim T, Kim SW. Bioreducible polymers for gene delivery. React Funct Polym. 2011;71:344–9. https://doi.org/10.1016/j.reactfunctpolym.2010.11.016 [ Google Scholar ].
Smith AE. Virial vectors in gene therapy, viral vectors in gene therapy. Annu Rev Microbiol. 1995;49:807–38 [ Google Scholar ].
Sung YK, Kim SW. The practical application of gene vectors in cancer therapy. Integrat Cancer Sci Therap. 2018;5:1–5 [ Google Scholar ].
CAS Google Scholar
Ibraheem D, Elaissari A, Fessi H. Gene therapy and DNA delivery systems. Int J Pharm. 2014;459:0–83 [ Google Scholar ].
Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–22 [ Google Scholar ].
Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Therap. 1998;80:35–47 [ Google Scholar ].
Nathaly S, Pere D, Anna C, Victor R, Salvador B. Oligopeptide-terminated poly(β-amino ester) s for highly efficient gene delivery and intracellular localization. Acta Biomater. 2014;10:2147–58 [ Google Scholar ].
Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, Chan YS, Wei L, Chin SM, Azad A, He AB-L, Leung AYH, Efficient RNA. drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9:2359. https://doi.org/10.1038/s41467-018-04791-8 Article No. [ Google Scholar ].
Wang F, Zhang W, Shen Y, Huang Q, Zhou D, Guo S, Efficient RNA. delivery by integrin-targeted glutathione responsive polyethyleneimine capped gold nanorods. Acta Biomater. 2015;23:136–46 [ Google Scholar ].
Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2008;8:129–38 [ Google Scholar ].
Arthanari Y, Pluen A, Rajendran R, Aojula H, Demonacos C. Delivery of therapeutic shRNA and siRNA by tat fusion peptide targeting BCR-ABL fusion gene in chronic myeloid leukemia cells. J Control Release. 2010;145:272–80 [ Google Scholar ].
Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70 [ Google Scholar ].
Merritt WM, Bar-Eli M, Sood AK. The dicey role of dicer: implications for RNAi therapy. Cancer Res. 2010;70:2571–4 [ Google Scholar ].
Mathiyalagan P, Sahoo S. Exosomes-based gene therapy for micro-RNA delivery methods. Mol Biol. 2017;152:139–52 [ Google Scholar ].
Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo . Am J Phys Lung Cell Mol Phys. 2017;312:110–21. https://doi.org/10.1152/ajplung.00423.2016 [ Google Scholar ].
Download references
Acknowlegements
This work had supported by the NIH Grant CA177932.
Author information
Authors and affiliations.
Department of Chemistry, College of Science, Dongguk University, Phildong-ro, Seoul, 04620, South Korea
Yong Kiel Sung
Department of Pharmaceutics and Pharmaceutical Chemistry, Center for Controlled Chemical Delivery, University of Utah, BPRB, Room 205, Salt Lake City, UT, 84112, USA
Yong Kiel Sung & Sung Wan Kim
You can also search for this author in PubMed Google Scholar
Contributions
Y. K. S and S. W. K discussed the review on recent advances in polymeric drug delivery systems and wrote the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yong Kiel Sung .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sung, Y.K., Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater Res 24 , 12 (2020). https://doi.org/10.1186/s40824-020-00190-7
Download citation
Received : 13 March 2020
Accepted : 19 May 2020
Published : 06 June 2020
DOI : https://doi.org/10.1186/s40824-020-00190-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drug delivery system
- Polymeric drug delivery
- Gene delivery system
- Viral vectors
- Non-viral vectors
Biomaterials Research
ISSN: 2055-7124
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Drug delivery
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Last »
- Chemoinformatics Follow Following
- Molecular modeling Follow Following
- Neurology and Psychiatry Follow Following
- Structural Biology Follow Following
- Targeted Drug Delivery Follow Following
- Drug Delivery System Follow Following
- Anticancer Drug Delivery Follow Following
- Biomaterials Follow Following
- Nanoparticles Follow Following
- Nanotechnology Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Journals
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
COMMENTS
The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on: • Controlled release • Bioavailability and drug absorption • Nanomedicines • Gene delivery • Tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.
Jun 1, 2023 · Combined drug delivery approach has been widely adopted in cancer research and therapy as a means to overcome multidrug resistance. It has been reported that combination drug delivery approach reduces therapeutic dosage as well as adverse reactions while efficiency and decrease in drug resistance are maintained [116].
Dec 3, 2024 · Drug delivery describes the method and approach to delivering drugs or pharmaceuticals and other xenobiotics to their site of action within an organism, with the goal of achieving a therapeutic ...
Mar 1, 2020 · Therefore, carrier-mediated transmembrane transport has received extensive attention. Drug transporters are recognized as a decisive factor for drug delivery and drug interaction. The research on the mechanism of uptake and efflux transporters lays a foundation for the development and improvement of drugs.
May 16, 2024 · Additionally, it critically analyzes the technological bottlenecks, current research challenges, and future trends in the application of novel drug delivery systems. The hydrophilic and ...
Current research into the role of engineered nanoparticles in drug delivery systems (DDSs) for medical purposes has developed numerous fascinating nanocarriers. This paper reviews the various conventionally used and current used carriage system to ...
Drug delivery using microbes is a diversified system compared to conventional route due to either live or vegetative state and are capable of self-propagating within tumor cells. The biggest challenge with microbial drug delivery is its distinctive regulatory requirements with additional safety, toxicity and manufacturing technology.
Dec 11, 2024 · In addition to comprehensive and cutting-edge research articles, the journal welcomes for consideration the following paper types: Critical reviews, Systematic reviews, Brief reports (cutting-edge short papers describing significant advances in an area of drug delivery of broad interest), Case report, Discussion (perspective), Data note and ...
Jun 6, 2020 · Background Polymeric drug delivery systems have been achieved great development in the last two decades. Polymeric drug delivery has defined as a formulation or a device that enables the introduction of a therapeutic substance into the body. Biodegradable and bio-reducible polymers make the magic possible choice for lot of new drug delivery systems. The future prospects of the research for ...
Extensive review pertaining specifically, to the patents relating to drug delivery across the CNS is currently available. However, many patents e.g. US63722506, US2002183683 etc., have been mentioned in a few articles. It is the objective of this article to expansively review drug delivery systems for CNS by discussing the recent patents available.