- Research article
- Open access
- Published: 27 September 2024

Strategies to improve the implementation of preventive care in primary care: a systematic review and meta-analysis
- Laura Heath ORCID: orcid.org/0000-0002-1628-1981 1 ,
- Richard Stevens ORCID: orcid.org/0000-0002-9258-4060 1 ,
- Brian D. Nicholson ORCID: orcid.org/0000-0003-0661-7362 1 ,
- Joseph Wherton ORCID: orcid.org/0000-0001-7701-4783 1 ,
- Min Gao ORCID: orcid.org/0000-0001-6196-7088 1 ,
- Caitriona Callan ORCID: orcid.org/0000-0002-5906-9542 1 ,
- Simona Haasova ORCID: orcid.org/0000-0002-3993-0462 1 , 2 &
- Paul Aveyard ORCID: orcid.org/0000-0002-1802-4217 1
BMC Medicine volume 22 , Article number: 412 ( 2024 ) Cite this article
1728 Accesses
1 Altmetric
Metrics details
Action on smoking, obesity, excess alcohol, and physical inactivity in primary care is effective and cost-effective, but implementation is low. The aim was to examine the effectiveness of strategies to increase the implementation of preventive healthcare in primary care.
CINAHL, CENTRAL, The Cochrane Database of Systematic Reviews, Dissertations & Theses – Global, Embase, Europe PMC, MEDLINE and PsycINFO were searched from inception through 5 October 2023 with no date of publication or language limits. Randomised trials, non-randomised trials, controlled before-after studies and interrupted time series studies comparing implementation strategies (team changes; changes to the electronic patient registry; facilitated relay of information; continuous quality improvement; clinician education; clinical reminders; financial incentives or multicomponent interventions) to usual care were included. Two reviewers screened studies, extracted data, and assessed bias with an adapted Cochrane risk of bias tool for Effective Practice and Organisation of Care reviews. Meta-analysis was conducted with random-effects models. Narrative synthesis was conducted where meta-analysis was not possible. Outcome measures included process and behavioural outcomes at the closest point to 12 months for each implementation strategy.
Eighty-five studies were included comprising of 4,210,946 participants from 3713 clusters in 71 cluster trials, 6748 participants in 5 randomised trials, 5,966,552 participants in 8 interrupted time series, and 176,061 participants in 1 controlled before after study. There was evidence that clinical reminders (OR 3.46; 95% CI 1.72–6.96; I 2 = 89.4%), clinician education (OR 1.89; 95% CI 1.46–2.46; I 2 = 80.6%), facilitated relay of information (OR 1.95, 95% CI 1.10–3.46, I 2 = 88.2%), and multicomponent interventions (OR 3.10; 95% CI 1.60–5.99, I 2 = 96.1%) increased processes of care. Multicomponent intervention results were robust to sensitivity analysis. There was no evidence that other implementation strategies affected processes of care or that any of the implementation strategies improved behavioural outcomes. No studies reported on interventions specifically designed for remote consultations. Limitations included high statistical heterogeneity and many studies did not account for clustering.
Conclusions
Multicomponent interventions may be the most effective implementation strategy. There was no evidence that implementation interventions improved behavioural outcomes.
Trial registration
PROSPERO CRD42022350912.
Peer Review reports
Smoking, obesity, alcohol intake, and physical inactivity bring forward the onset of chronic disease, multimorbidity, and premature death. Compared to individuals with no behavioural risk factors, those with 2 or more risk factors (smoking, obesity, alcohol intake and physical inactivity) can expect to live on average 12 years fewer [ 1 ]. In 2015, 30.3% and 15.5% of global disease burden were accounted for by behavioural factors and metabolic factors, respectively [ 2 ]. Differences in the prevalence of these risk factors explain a substantial portion of the health gap between those from affluent and deprived areas [ 3 , 4 ]. One way health systems address this is through preventive healthcare. This includes supporting behaviour change via brief opportunistic interventions and referral to further support [ 5 , 6 ]. Systematic reviews of randomised trials and modelling from their results show that opportunistic screening for and intervention to support behaviour change is effective and cost-saving for smoking cessation [ 7 , 8 ]; effective and cost-effective for reducing hazardous drinking [ 9 , 10 ]; effective and cost-effective for weight loss in obesity [ 11 , 12 ]; effective and may be cost-effective for physical inactivity [ 5 , 13 ].
These behaviour changes reduce the development of type 2 diabetes, cardiovascular disease, cancer and premature mortality [ 14 ]. They have been shown to be feasible in primary care and equitable in their impact [ 15 ]. Increasing preventive care delivery can also reduce the environmental impact of healthcare and support the transition to more sustainable healthcare systems [ 16 ]. Optimising the implementation of these evidence-based interventions is a health system priority [ 17 ]. However, the rate of intervention by primary healthcare professionals, who are well-placed to deliver them, is low [ 18 , 19 , 20 ]. For example, in the UK in 2020, the rate of advice for weight management (8 events per hundred patients per year), physical inactivity (4 events per hundred patients per year) and excessive alcohol intake (4 events per hundred patients per year) was low compared to the prevalence of overweight or obesity (approximately 60%), of physical inactivity (approximately 30%) and of harmful alcohol consumption (approximately 20%) [ 21 , 22 , 23 ]. Given their effectiveness and cost-effectiveness, governments and health systems have attempted to increase the implementation of this type of preventive healthcare [ 17 , 24 ]. We therefore conducted a systematic review and meta-analysis to examine the effectiveness of different implementation strategies (Table 1 ) compared to usual care, in adults in a primary healthcare setting to increase both process and behavioural outcomes for smoking, obesity, excessive alcohol consumption and physical inactivity.
The way primary health care is being delivered is also changing as more consultations are being delivered remotely (via telephone, video, email or text message), and this may affect implementation efforts [ 25 , 26 ]. Therefore, we also aimed to examine the effectiveness of implementation strategies in this current context.
A full protocol was prospectively published on the International Prospective Register of Systematic Reviews (PROSPERO) [ 27 ]. This followed the Cochrane Effective Practice and Organisation of Care (EPOC) guidelines for an implementation systematic review and is reported according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [ 28 , 29 ].
Data sources and searches
We searched the Cumulated Index in Nursing and Allied Health Literature (CINAHL), The Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Database of Systematic Reviews, Dissertations & Theses – Global, Excerpta Medica Database (Embase), Europe PubMed Central (PMC), Medical Literature Analysis and Retrieval System Online (MEDLINE) and PsycINFO for studies until 5th October 2023. The references of included studies were manually searched for studies missed in the database search. The complete database search strategy is included in Additional file 1.
Study selection
The population considered for inclusion were adult patients seeking primary health care, where interventions for behaviour change happen opportunistically. If adolescents were also included in the study population, we only analysed the participants over the age of 18. If it was not possible to separate those under 18 from adult participants, we only included the study if the average age of participants was over 18 years. In this review, we use the National Health Service (NHS) England definition of primary care, including the general practice multidisciplinary team, community pharmacy, dental and optometry services [ 30 ]. When it was unclear whether a study was conducted in primary care, a decision was made in consultation with the wider research team which included three primary care physicians, considering whether this was the first point of contact for the patient in the healthcare system. We included cluster randomised trials (cRT), cluster non-randomised trials (cNRT), randomised trials (RT), controlled before-after studies (CBA) and interrupted time series (ITS) studies.
Exclusion criteria were adults seeking care for established disease, e.g. weight loss as a treatment for type 2 diabetes, and people who were receiving palliative care. There were no date or language restrictions.
Interventions included were those both at the health system and health professional level and these were compared to usual care. We included interventions that used one of ten implementation strategies (Table 1 ) to encourage action on smoking, poor diet, alcohol consumption or physical inactivity. This taxonomy of implementation strategies was adapted from the taxonomy used by the Cochrane EPOC group [ 31 , 32 ]. A similar approach has been used in another study looking at implementation strategies to optimise care for type 2 diabetes [ 33 ].
After deduplication, two reviewers independently screened title and abstracts and then full-text records against prespecified eligibility criteria using a decision flowchart. Covidence screening software (Veritas Health Innovation) was used for deduplication and title, abstract, and full-text screening [ 34 ].
Data extraction and quality assessment
Data were then extracted independently by two reviewers using a piloted Microsoft Excel spreadsheet. Extracted information included baseline characteristics, study design, intervention characteristics and outcome measures. Risk of bias was independently assessed by two authors using the Cochrane EPOC risk of bias tool for the appropriate study design. Disagreements were resolved by the wider team for review.
Data synthesis and analysis
In line with Donabedian’s three-component approach for measuring the quality of care, the main outcomes were measures that record changes in process (e.g. referral to further support) and behavioural outcome (e.g. smoking cessation or weight loss) of preventive care [ 35 ]. Outcomes were grouped by predominant mode of intervention e.g., clinician education or team changes. Outcomes were extracted at 1 year, or the closest measurement to this. Secondary outcomes included patient acceptability and satisfaction with the intervention; healthcare professional acceptability and satisfaction with the intervention; resource use; equity impact, and adverse effects.
Where more than one health behaviour was reported we prioritised a summary statistic of the effect of the intervention on combined health behaviours, the primary outcome of the study, or if neither of these were present, the first reported health behaviour. If more than one process outcome was reported, we took the most distal outcome, e.g. if advice given and referral made were both reported, we used the referral data. We included studies that reported changes in health behaviours (e.g. diet) and outcomes of health behaviours (e.g. weight) and grouped these together for the behavioural outcomes meta-analysis.
We extracted continuous and dichotomous outcomes, converting these where required from a standardised effect measure to an odds ratio (OR) using an adapted Chinn’s method [ 36 ]. Where a study did not correct for clustering, we adjusted the width of the confidence interval as described in the Cochrane handbook [ 37 ]. The upper quartile intracluster correlation coefficient (ICC) was selected from the included studies. This conservative approach also reduces the influence of outlier values. When the number of cases in the intervention or control group was zero, the Peto method was used to calculate an OR. Calculation details can be found in Additional file 2.
Where an adjusted hazard ratio, incident rate ratio or risk ratio was given by the study, this was taken as a conservative estimate of the odds ratio. If two or more intervention groups existed, we selected the intervention group that most closely represented the implementation strategy of interest. For example, we used the training workshop and usual care arms, and not the free patient education material arm in the Kottke et al. study [ 38 ]. Where there were intervention arms of different intensities, these were combined into a single intervention arm. Some studies provided insufficient information to calculate a standardised effect measure. In these cases, the authors were contacted and if no further information was provided, these results were synthesised narratively.
We used random effects meta-analysis to pool study outcomes, given that the true effect of preventive interventions is likely to differ across contexts and health behaviours. Sensitivity analysis (Additional file 3: Fig. S1–S2) used two further models to pool the study outcomes. Firstly, the Hartung-Knapp-Sidik-Jonkman (HKSJ) model variance correction was applied to the standard DerSimonian-Laird model; optionally without truncation of correction factor at 1, to reduce the risk of poor coverage of 95% confidence intervals (CIs). Secondly, the inverse variance heterogeneity (IVhet) model, for meta-analysis of heterogenous studies [ 39 , 40 ]. Meta-analysis was conducted in Stata, version 14.2 (StataCorp) using the admetan command. The full Stata code is available in Additional file 4. Forest plots were used to display the results of meta-analysis. The I 2 statistic and 95% prediction intervals were calculated to assess heterogeneity.
Additional sensitivity analyses were conducted where possible, excluding firstly studies at high risk of bias, secondly studies where data had to be imputed (Additional file 3: Fig. S3–S4), and lastly when outcomes of health behaviours were reported (e.g. weight) rather than the health behaviour directly (e.g. diet) (Additional file 3: Fig. S5). Subgroup analysis considered each health behaviour separately (Additional file 3: Fig. S6–S7). These analyses were completed where there was more than one study able to be meta-analysed in each subgroup.
Role of the funding source
This review was funded by the Wellcome Trust who had no role in the design of the study or analysis or interpretation of the data.
Figure 1 shows the flow through the study. The search identified 22,545 unique study titles. After screening, 456 full texts were assessed for eligibility and 85 studies were included.
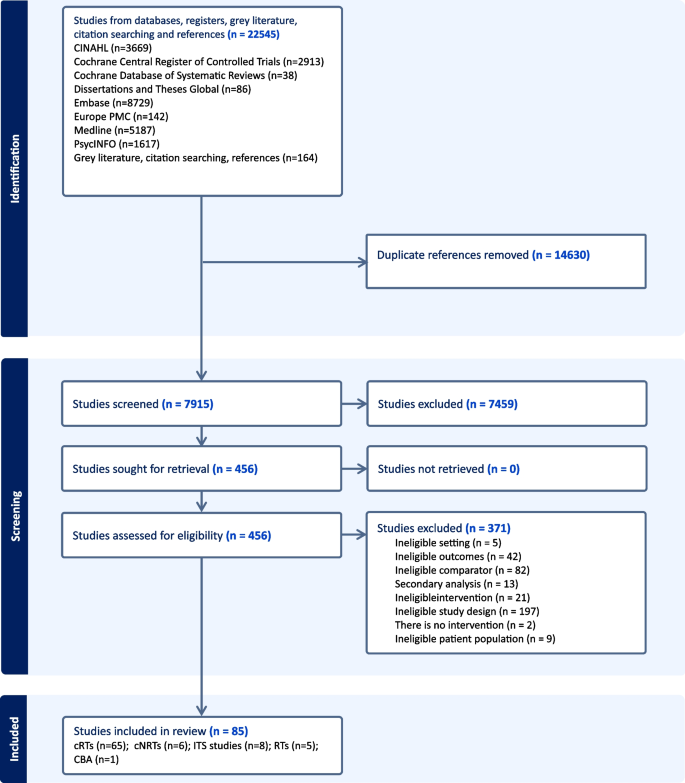
PRISMA flow diagram
Study characteristics
Of the 85 included studies (Additional file 4: Table S1), [ 38 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 ] 65 were cRTs, 6 were cNRTs, 8 were ITS studies, 5 were RTs and 1 was a CBA study. The 71 cluster trials had a total of 3713 clusters and 4,210,946 participants. The randomised trials had a total of 6748 participants, the interrupted time series studies used data from 5,966,552 participants 176,061 participants in 1 CBA study. Forty-two studies focussed on smoking, 19 on alcohol, 7 on obesity (poor diet), 2 on physical activity and 15 on multiple health behaviours. The average age of patients and healthcare professionals was 49 and 43 years, respectively. Forty-eight per cent of patients and 45% of healthcare professionals were male. Of the small number of studies that reported ethnicity, 67% of patients and 69% of healthcare professionals were White. The most common countries were the USA ( n = 42), Europe ( n = 15) and the UK ( n = 13). The mean (standard deviation) follow-up time for ITS studies was 44 (42) months and for the other study types was 10 (6) months.
No studies examined the impact of preventive care management interventions or audit and feedback interventions. Three studies investigated the impact of team changes; 5 studies investigated the electronic patient registry; 5 studies investigated the facilitated relay of information; 1 study investigated continuous quality improvement; 37 studies investigated clinician education interventions; 9 investigated clinical reminders; 7 investigated financial incentives and 18 investigated multi-component interventions. Of the studies, 68 were included in meta-analyses and 17 studies were synthesised narratively. No studies examined implementation strategies specifically for remote consultations in primary care.
Risk of bias
Risk of bias was assessed to be low in 25 studies, unclear in 24 studies and high in 36 studies (Tables 2 and 3 ). Removing studies at high risk of bias in the sensitivity analysis did not significantly change the meta-analysis results (Additional file 3: Fig S3–S4).
Process outcomes
Sixty studies were included in the process outcome meta-analysis from 7 intervention groups: 7 clinical reminder studies; 29 clinician education studies, 4 electronic patient registry studies, 4 facilitated relay of information studies, 2 financial incentive studies, 12 multi-component studies and 2 team change studies (Fig. 2 ).
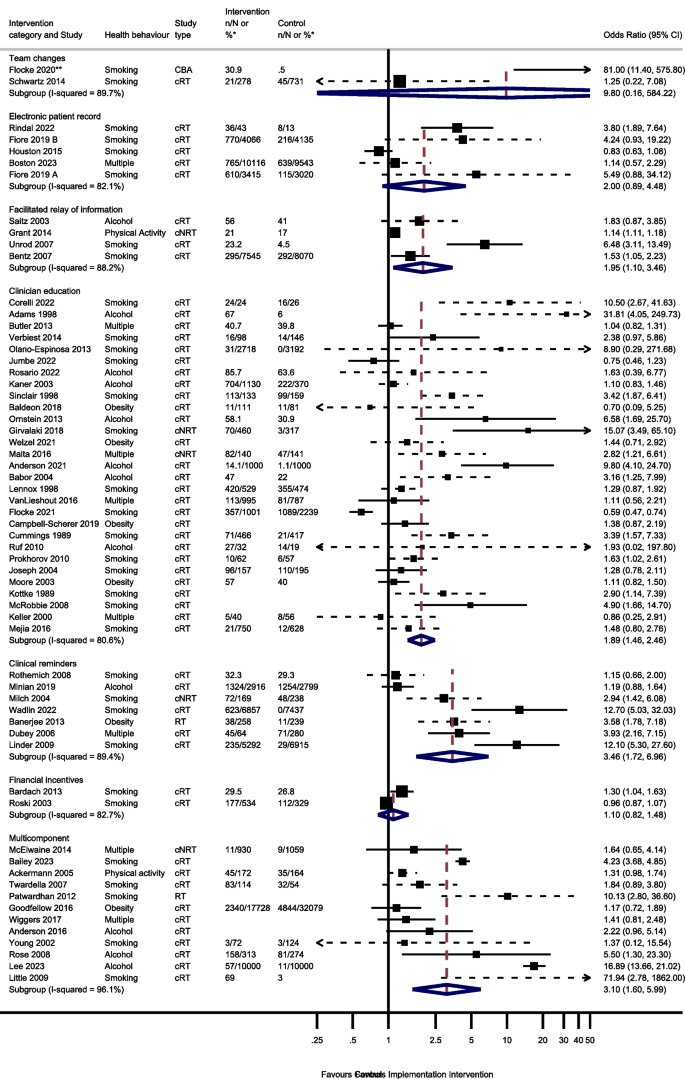
Odds of improving process outcomes between implementation interventions and control groups using random effects meta-analysis
*n/N or % given where available in the paper. ** Flocke study estimate not visible for reasons of scale. Note: Dashed line indicates studies for which approximate data had to be used
Three studies reported the effect of team changes on process outcomes. One ITS study was not included in the meta-analysis. This study found team changes resulted in a significant increase in the number of people receiving an appropriate weight management referral or smoking cessation intervention [ 44 ]. The meta-analysis of the remaining 2 studies reported imprecise results (OR 9.80, 95% CI 0.16–584.22, I 2 = 89.7%, 95% CI 62–97%).
Four studies examined the effect of changing the electronic patient record. One of the 4 electronic patient record studies had 2 separate analyses for 2 different healthcare systems [ 66 ]. There was no evidence that amending the electronic patient record increased preventive processes (OR 2.00; 95% CI 0.89–4.48, I 2 = 82.1%, 95% CI 59–92%).
Five studies reported the effect of the facilitated relay of information on preventive care, with 4 included in the meta-analysis. One study was unable to complete clinician and practice level analyses because the sample was too small [ 128 ]. There was evidence that facilitated relay of information interventions significantly increased preventive processes (OR 1.95, 95% CI 1.10–3.46, I 2 = 88.2%, 95% CI 72–95%).
Thirty-one studies examined the effects on process outcomes of clinician education interventions. Two studies were unable to be included in the analysis [ 69 , 80 ]. One study showed no evidence of a significant difference between intervention and control groups being signposted to quitline services by pharmacy staff [ 80 ]. The second study found that training and support for GPs significantly increased the rate of implementation of brief interventions for alcohol [ 69 ]. Meta-analysis of the remaining 29 studies showed a significant increase in preventive process outcomes (OR 1.89, 95% CI 1.46–2.46, I 2 = 80.6%, 95% CI 73–86%).
Seven of the 8 clinical reminder studies with process outcomes were able to be meta-analysed. These showed a statistically significant increase in health process outcomes (OR 3.46, 95% CI 1.72–6.96, I 2 = 89.4%, 95% CI 81–94%). One study not included in the meta-analysis reported that there were no significant differences between groups in changes in the percentages of patients who had a nutrition counselling visit when reminders and alerts were added to the records of patients with overweight or obesity [ 135 ].
Six studies reported the effect of financial incentives on process outcomes. There were differences in the way that results were reported for four ITS studies, precluding meta-analysis. Three reported statistically significant positive associations between financial incentives and greater alcohol and smoking advice/interventions, [ 100 , 122 , 136 ] and one reported a positive association between the financial incentive and clinicians’ alcohol advice or intervention without commenting on statistical significance [ 106 ]. Two cRTs reported process outcomes [ 55 , 111 ]. There was no evidence from the meta-analysis of these studies that financial incentives increased the delivery of preventive processes (OR 1.10, 95% CI 0.82–1.48, I 2 = 82.7%, 95% CI 27–96%).
Seventeen multicomponent intervention studies reported process outcomes, of which 12 were included in the meta-analysis. Two ITS studies not included in the meta-analysis found that multicomponent interventions were associated with an increase in the number of people screened for tobacco use or receiving a smoking cessation intervention, [ 52 ] and an increase in alcohol recording [ 125 ]. One study not included in the meta-analysis found a statistical increase in the percentage of tobacco users who received a cessation intervention [ 85 ]. However, another study not included in the meta-analysis reported no evidence that patients were more likely to receive behavioural advice or referral at follow-up for any health behaviour, [ 74 ] and another found no evidence for a change in alcohol screening after implementing a multicomponent intervention [ 125 ]. Of the 12 studies included in the meta-analysis, there was evidence that multicomponent interventions increased the process outcomes (OR 3.10, 95% CI 1.60–5.99, I 2 = 96.1%, 95% CI 95–97%).
Sensitivity and subgroup analysis
Sensitivity analysis showed that multicomponent interventions were the only category of intervention that showed evidence of increased process outcomes in all 3 meta-analysis models (random effects, HKSJ, and IVhet) (Additional file 3: Fig. S1). Removing studies at high risk of bias or studies that required imputed data did not significantly change the results (Additional file 3: Fig. S3). Subgroup analysis showed that there was no evidence that clinician education or multicomponent interventions that targeted multiple health behaviours increased process outcomes (Additional file 3: Fig. S6).
Behavioural outcomes
Thirty-nine studies reported behavioural outcomes and were able to be meta-analysed, assessing the effect of 5 intervention modes. This included 15 studies of clinician education; 3 studies of clinical reminders; 2 electronic patient registry studies; 3 facilitated relay of information studies, and 4 studies of multicomponent interventions (Fig. 3 ).
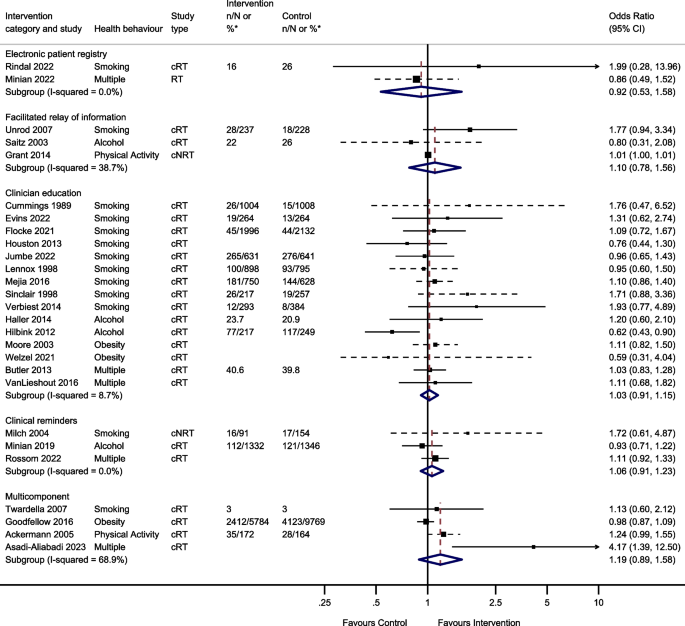
Odds of improving behavioural outcomes between implementation interventions and control groups using random effects meta-analysis
*n/N or % given where available in the paper. Note: Dashed line indicates studies for which approximate data had to be used
Two studies reported the effect of team changes on behavioural outcomes. These were unable to be meta-analysed due to differences in the reporting of data. One found that individuals who were in the intervention group were significantly more likely than controls to have a lower body mass index (BMI) and to have quit smoking at the end of the follow-up period, [ 44 ] the other found no evidence of a difference in self-reported smoking cessation at 8 months [ 117 ].
Two studies investigated the effect of changes to the electronic patient record on behavioural outcomes and were meta-analysed. There was no evidence that changes to the electronic patient registry improved behavioural outcomes (OR 0.92, 95% CI 0.53–1.58, I 2 = 0.0%, 95% CI 0–100%).
Four studies reported the effect of facilitated relay of information on beneficial behavioural outcomes. One study was unable to complete clinician and practice level analyses because the sample was too small [ 128 ]. Three studies included in the meta-analysis found no evidence that facilitated relay of information improved behavioural outcomes (OR 1.10, 95% CI 0.78–1.56, I 2 = 38.7%, 95% CI 0–81%).
One study investigated the effect of continuous quality improvement on smoking outcomes [ 133 ]. There was no evidence of a difference in smoking cessation between the intervention and the control groups (OR 0.90, 95% CI 0.58–1.39).
Twenty studies reported the effect of clinician education on behavioural outcomes. Five were unable to be included in the meta-analysis due to insufficient information. One study showed a significant increase in smoking cessation in the intervention group compared to controls at 1 year [ 101 ]. Another study found the intervention group had a greater reduction in alcohol dependence score during follow-up compared to the control group [ 105 ]. However, three studies found no evidence of a difference in rates of smoking cessation, alcohol dependence score or difference in BMI/ weight between intervention and control groups during follow-up [ 38 , 53 , 115 ]. The meta-analysis showed there was no evidence that clinician education (OR 1.03, 95% CI 0.91–1.15, I 2 = 8.7%, 95% CI 0–46%) improved behavioural outcomes.
Four studies reported the effect of clinical reminders on beneficial behavioural outcomes. One study not included in the meta-analysis due to insufficient data showed no evidence of a difference in weight loss between intervention and control groups [ 50 ]. The remaining 3 studies included in the meta-analysis showed no evidence that clinical reminders improved behavioural outcomes (OR 1.06, 95% CI 0.91–1.23, I 2 = 0.0%, 95% CI 0–11%).
Two studies reported the effect of financial incentives on behavioural outcomes. One was an ITS study, and the other was a cRT. Due to the difference in reporting of data, these were unable to be meta-analysed. One reported that financial incentives were significantly associated with reduced smoking, but not with reduced BMI or alcohol consumption in the 6 years following the introduction of the financial incentive [ 65 ]. The other showed there was no evidence of an effect of financial incentives on smoking cessation at 6 months [ 111 ].
Four studies reported the effect of multicomponent interventions. There was no evidence that multicomponent interventions improved behavioural outcomes (OR 1.19, 95% CI 0.89–1.58, I 2 = 68.9%, 95% CI 10–89%).
Sensitivity analysis showed no significant difference in meta-analysis results using different models (random effects, HKSJ, and IVhet) (Additional file 3: Fig. S2), when excluding studies at high risk of bias, or studies that used imputed data (Additional file 3: Fig. S4). Due to the small number of studies in the meta-analysis, subgroup analysis was only conducted for clinician education studies. There was no evidence that the effect of clinician education varied between smoking, alcohol, obesity, or multiple behavioural outcomes (Additional file 3: Fig. S7). Removing the three studies that measured the outcome of a health behaviour (weight) rather than the health behaviour directly (diet) did not change the result of the meta-analysis (Additional file 3: Fig. S5) [ 71 , 98 , 130 ].
Secondary outcomes
Most studies did not report data on our secondary outcomes. Three studies reported no adverse effects of the intervention [ 41 , 64 , 82 ]. Thirteen studies reported that training received was useful, relevant, or increased healthcare professional confidence and self-esteem [ 61 , 70 , 71 , 75 , 82 , 90 , 104 , 105 , 108 , 120 , 127 , 130 , 134 ]. One electronic health record study commented that eReferral had good reach amongst people without health insurance, and this could help reduce health inequalities [ 66 ]. However, another study described how people from deprived communities and smokers were less likely to take up the offer of an additional health check [ 44 ].
One study reported on barriers to weight management in follow-up interviews. Healthcare professionals described how too many clinical reminders were counterproductive [ 50 ]. A clinical education smoking study in community pharmacies described time constraints, privacy, and part-time staff as barriers to maintaining an accurate clinical record [ 118 ]. Healthcare professionals in another clinical education study questioned whether in-depth training for weight management was feasible against other competing clinical demands [ 98 ].
Three studies presented data relevant to the cost-effectiveness of the intervention. One calculated the additional cost of clinician-specific feedback at US$65 per estimated quit [ 128 ]. Another clinical education study targeting smoking cessation, found the intervention incremental cost per life year gained after 6 months was €969 [ 101 ]. Finally, a clinical education study for excess alcohol found a similar cost-effectiveness ratio between control and intervention practices [ 83 ].
There was some evidence that many implementation strategies including clinical reminders, clinician education, facilitated relay of information and multicomponent interventions increased the occurrence of preventive processes of care. Multicomponent interventions were the most robust in sensitivity analysis. There was some evidence in subgroup analysis that implementation strategies that target multiple behaviours may be less effective than those that target single behaviours. However, there was no consistent evidence that these process changes translated into improved behavioural outcomes.
Strengths and limitations
Our search strategy was thorough, including 8 databases, grey literature, references, and citation searching, with no date or language constraints. The resulting sample is large with data from over 10 million participants. Included studies came from North America, Europe, South America, the Middle East, and Australia so our results are relevant to many healthcare systems.
Dividing interventions into implementation categories allows the results to be relevant to policy makers and public health professionals, although for a few studies, the predominant implementation strategy was not clear. We resolved these through consensus of all investigators. We followed PRISMA guidance throughout this study (Additional file 6 and Additional file 7).
Many of our studies had a high risk of bias. This was partly due to our decision to include a greater range of study designs and include non-randomised studies. This decision was made as some interventions e.g., financial incentives implemented across an entire health care system cannot practically be randomised in a traditional trial setting. There was also high heterogeneity, especially in the primary analyses. This is not unexpected as, although interventions were of a similar type, they differed in intensity, duration, delivery, and format. In addition, studies were conducted in different countries, healthcare systems with different methods of usual care, and amongst different population demographics, which also likely contributed to the high heterogeneity. In the presence of such high heterogeneity, we considered it useful to conduct meta-analysis and examine statistical significance, but the individual point estimates should not be over-interpreted. Random-effects meta-analysis allows for differences in the intervention effect between studies and provides an estimate of the average intervention effect [ 137 ]. We also calculated prediction intervals for each meta-analysis conducted (Additional file 8: Table S1). These confirm the high levels of heterogeneity in the analysis, with a range of ORs plausible for different settings or populations. We did not formally assess for publication bias or other small study effects. We think it unlikely that unreported studies or results would change our conclusions. Only two studies reported information about how the intervention effectiveness differed between socioeconomic groups. Future studies should collect this information to understand which population groups may benefit most.
We had to impute several calculations as many studies had not accounted for clustering, or we had to calculate an OR from available data. Whilst these established methods, they introduce another opportunity for error. Resulting CIs were often wide, reflecting the uncertainty in data from single trials.
When analysing behavioural outcomes, we combined studies that targeted both behaviours directly and those that targeted outcomes of behaviours. One study measured alcohol dependence rather than consumption using the Alcohol Use Disorders Identification Test (AUDIT) score [ 76 ]. However, this scoring system is strongly correlated with alcohol consumption, and so we retained this study in the meta-analysis [ 138 , 139 ]. Another study used a combined metric of smoking status, alcohol consumption, physical activity and diet [ 58 ]. We also retained this study in the meta-analysis as most of the measures used (smoking status, alcohol consumption and physical activity) were used by the other studies. It seems unlikely that including these studies would change the conclusion that there was no evidence of effect. Furthermore, a post hoc sensitivity analysis that excluded three studies that measured the outcome of a health behaviour (weight) rather than the health behaviour (diet) directly, did not change the result of the meta-analysis that there was no evidence of effect [ 71 , 98 , 130 ].
The observed difference in process and behavioural outcomes may be because outcomes can be measured more precisely in proximal (process) outcomes, behavioural outcomes take time to emerge, and many studies did not have sufficient follow-up periods to capture changes in patient behaviour. Furthermore, process outcomes are recorded at the time of consultation, while changes in behaviour may have occurred without the patient returning and/or the clinician asking about behaviour and recording the change at a subsequent consultation. Moreover, increases in the recording of processes of care may reflect that clinicians start recording activity that was previously unrecorded. If so, increases in processes of care may occur while changes in patient behaviour would not be expected to increase. This is particularly likely to occur where the intervention is a financial incentive. However, trial data has shown that genuine increases in process outcomes does translate into beneficial behavioural outcomes [ 11 ].
Comparison with existing literature
To our knowledge, this is the first systematic review to investigate implementation strategies for preventive healthcare. Other studies have focussed on quality improvement strategies in chronic disease management or fall prevention. For example, systematic reviews investigating quality improvement strategies in diabetes care found that multicomponent QI programmes may achieve meaningful population improvements for most immediate diabetes outcomes and that interventions at the health system or patient level may be more effective than interventions at the health professional level [ 33 , 140 ]. A comprehensive systematic review looking at falls prevention found evidence that team changes and multicomponent interventions may reduce falls [ 141 ]. Another review reported that clinician education and patient reminders and education were the most effective strategies for reducing systolic and diastolic blood pressure respectively [ 142 ]. Our review supports these findings for the implementation of preventive healthcare in primary care. This suggests that quality improvement strategies may be generalisable across clinical targets. However, due to the high heterogeneity observed in this review, the results should be interpreted cautiously. Future research should collect data about which population subgroups may benefit most from these interventions.
Like our review, previous systematic reviews have found no evidence that financial incentives improve the quality of healthcare, with evidence of small benefits at best, and urged caution when policy makers are considering introducing new incentives [ 143 , 144 ]. Although our study was not able to meta-analyse the effectiveness of quality improvement strategies, another review found the Plan-Do-Study-Act (PDSA) improved the quality of care in most included studies, despite many studies not adhering to the key method features [ 145 , 146 ].
Future implications
A limitation of this review was the quality of the included studies; many studies used a cluster design but did not account for clustering in their analysis. Studies should ensure that studies are powered to detect modest effects on behavioural outcomes, account for clustering and allow sufficient follow-up time to accrue sufficient events to detect changes in behavioural outcome as well as process outcomes.
No studies looked specifically at implementation strategies in remote consultations. As the delivery of primary care is changing, future research should consider whether these implementation strategies are effective across consultation modalities.
These results show that a broad suite of intervention strategies, and in particular multicomponent interventions may improve processes of preventive care. However, there is no evidence that these strategies improve patient outcomes through behaviour change, such as smoking cessation, increased physical activity, or reduced bodyweight or alcohol consumption. There is evidence that introducing population health interventions affect individuals’ attempts to change their behaviour and the success of those attempts to change [ 147 , 148 ]. Consequently, it may be helpful for policy makers to combine multicomponent implementation strategies to increase the delivery of preventive healthcare in primary care with population-level interventions to maximise health benefits.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Abbreviations
Quality improvement
International Prospective Register of Systematic Reviews
Effective Practice and Organisation of Care
Preferred Reporting Items for Systematic reviews and Meta-Analysis
Cumulated Index in Nursing and Allied Health Literature
The Cochrane Central Register of Controlled Trials
Excerpta Medica Database
PubMed Central
Medical Literature Analysis and Retrieval System Online
National Health Service
Cluster randomised trial
Cluster non-randomised trial
Randomised trial
Controlled before-after
Interrupted time series
Intracluster correlation coefficient
Hartung-Knapp-Sidik-Jonkman
Confidence interval
Inverse variance heterogeneity
Body mass index
Alcohol Use Disorders Identification Test
Plan-Do-Study-Act
Zaninotto P, Head J, Steptoe A. Behavioural risk factors and healthy life expectancy: evidence from two longitudinal studies of ageing in England and the US. Sci Rep. 2020;10(1):6955.
Article CAS PubMed PubMed Central Google Scholar
Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–724.
Marteau TM, Rutter H, Marmot M. Changing behaviour: an essential component of tackling health inequalities. BMJ. 2021;372:n332.
Article PubMed PubMed Central Google Scholar
Everest G, Marshall L, Fraser C, Briggs A. Addressing the leading risk factors for ill health A review of government policies tackling smoking, poor diet, physical inactivity and harmful alcohol use in England. https://www.health.org.uk/sites/default/files/upload/publications/2022/Risk%20factors_Web_Final_Feb.pdf : The Health Foundation; 2022.
U. S. Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Coker TR, et al. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors: us preventive services task force recommendation statement. Jama. 2022;328(4):367–74.
Article Google Scholar
National institute for Health and Care Excellence (NICE). Behaviour change: individual approaches https://www.nice.org.uk/guidance/ph49/chapter/recommendations2014
Maciosek MV, LaFrance AB, Dehmer SP, McGree DA, Xu Z, Flottemesch TJ, et al. Health Benefits and Cost-Effectiveness of Brief Clinician Tobacco Counseling for Youth and Adults. Ann Fam Med. 2017;15(1):37–47.
U. S. Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US preventive services task force recommendation statement. Jama. 2021;325(3):265–79.
Purshouse RC, Brennan A, Rafia R, Latimer NR, Archer RJ, Angus CR, et al. Modelling the cost-effectiveness of alcohol screening and brief interventions in primary care in England. Alcohol Alcohol. 2013;48(2):180–8.
Article PubMed Google Scholar
U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US preventive services task force recommendation statement. Jama. 2018;320(18):1899–909.
Aveyard P, Lewis A, Tearne S, Hood K, Christian-Brown A, Adab P, et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomised trial. Lancet. 2016;388(10059):2492–500.
Retat L, Pimpin L, Webber L, Jaccard A, Lewis A, Tearne S, et al. Screening and brief intervention for obesity in primary care: cost-effectiveness analysis in the BWeL trial. Int J Obes (Lond). 2019;43(10):2066–75.
Gc VS, Suhrcke M, Hardeman W, Sutton S, Wilson ECF, Very Brief Interventions Programme T. Cost-effectiveness and value of information analysis of brief interventions to promote physical activity in primary care. Value Health. 2018;21(1):18–26.
Posadzki P, Pieper D, Bajpai R, Makaruk H, Konsgen N, Neuhaus AL, et al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health. 2020;20(1):1724.
Graham J, Tudor K, Jebb SA, Lewis A, Tearne S, Adab P, et al. The equity impact of brief opportunistic interventions to promote weight loss in primary care: secondary analysis of the BWeL randomised trial. BMC Med. 2019;17(1):51.
Mortimer F. The sustainable physician. Clin Med (Lond). 2010;10(2):110–1.
NHS England and NHS Improvment. The NHS Long Term Plan. https://www.longtermplan.nhs.uk/publication/nhs-long-term-plan/2019 .
Laddu D, Ma J, Kaar J, Ozemek C, Durant RW, Campbell T, et al. Health Behavior Change Programs in Primary Care and Community Practices for Cardiovascular Disease Prevention and Risk Factor Management Among Midlife and Older Adults: A Scientific Statement From the American Heart Association. Circulation. 2021;144(24):e533–49.
Rubio-Valera M, Pons-Vigués M, Martínez-Andrés M, Moreno-Peral P, Berenguera A, Fernández A. Barriers and Facilitators for the Implementation of Primary Prevention and Health Promotion Activities in Primary Care: A Synthesis through Meta-Ethnography. PLoS ONE. 2014;9(2):e89554.
Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study. BMJ Open. 2015;5(1):e006642.
Nuffield Trust. Obesity; 2022. https://www.nuffieldtrust.org.uk/resource/obesity .
NHS Digital. Health Survey for England, 2021 part 1 https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2021/part-3-drinking-alcohol2022
Heath L, Ordonez-Mena JM, Aveyard P, Wherton J, Nicholson BD, Stevens R. How has the COVID-19 pandemic affected the delivery of preventive healthcare? An interrupted time series analysis of adults in English primary care from 2018 to 2022. Prev Med. 2024;181:107923.
Litt J. RACGP Guidelines for preventive activities in general practice 9th edition. 2016.
Huibers L, Moth G, Carlsen AH, Christensen MB, Vedsted P. Telephone triage by GPs in out-of-hours primary care in Denmark: a prospective observational study of efficiency and relevance. Br J Gen Pract. 2016;66(650):e667–73.
Pearl R. Kaiser Permanente Northern California: current experiences with internet, mobile, and video technologies. Health Aff. 2014;33(2):251–7.
Heath LN, B.; Aveyard, P. Strategies to improve the implementation of preventative care in primary care: a systematic review. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022350912 : PROSPERO; 2022. Contract No.: CRD42022350912.
Cochrane Effective Practice and Organisation of Care. EPOC resources for review authors https://epoc.cochrane.org/resources/epoc-resources-review-authors2021
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74(9):790–9.
NHS England. Primary care services https://www.england.nhs.uk/get-involved/get-involved/how/primarycare/ [
Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Medical care. 2001;39(8 Suppl 2):Ii2–45.
CAS PubMed Google Scholar
AHRQ Technical Reviews. In: Shojania KG, McDonald KM, Wachter RM, Owens DK, editors. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol 1: Series Overview and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2004.
Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252–61.
Veritas Health Innovation. Covidence systematic review software. Australia: Melbourne; 2024.
Google Scholar
Donabedian A. Evaluating the Quality of Medical Care. Milbank Q. 2005;83(4):691–729.
Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–31.
Article CAS PubMed Google Scholar
Higgins JE, Li T, S. Li T. Chapter 23: Including variants on randomized trials. Cochrane Handbook for Systematic Reviews of Interventions version 64. 2023.
Kottke TE, Brekke ML, Solberg LI, Hughes JR. A Randomized Trial to Increase Smoking Intervention by Physicians: Doctors Helping Smokers. Round I JAMA. 1989;261(14):2101–6.
Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15(1):99.
SAR, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity mode. Contemp Clin Trials. 2015;45:130–8.
Ackermann RT, Deyo RA, LoGerfo JP. Prompting primary providers to increase community exercise referrals for older adults: a randomized trial. J Am Geriatr Soc. 2005;53(2):283–9.
Adams A, Ockene JK, Wheller EV, Hurley TG. Alcohol counseling: physicians will do it. J Gen Intern Med. 1998;13(10):692–8.
Alageel S, Gulliford MC. Effect of the NHS Health Check programme on cardiovascular disease risk factors during 6 years’ follow-up: matched cohort study. The Lancet. 2018;392(Supplement 2):S17.
Alageel S, Gulliford MC. Health checks and cardiovascular risk factor values over six years’ follow-up: Matched cohort study using electronic health records in England. PLoS Med. 2019;16(7): e1002863.
Anderson P, Bendtsen P, Spak F, Reynolds J, Drummond C, Segura L, et al. Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the Optimizing Delivery of Health Care Intervention (ODHIN) five-country cluster randomized factorial trial. Addiction. 2016;111(11):1935–45.
Anderson P, Manthey J, Llopis EJ, Rey GN, Bustamante IV, Piazza M, et al. Impact of Training and Municipal Support on Primary Health Care-Based Measurement of Alcohol Consumption in Three Latin American Countries: 5-Month Outcome Results of the Quasi-experimental Randomized SCALA Trial. J Gen Intern Med. 2021;36(9):2663–71.
Asadi-Aliabadi M, Karimi SM, Mirbaha-Hashemi F, Tehrani-Banihashemi A, Janani L, Babaee E, et al. Motivating non-physician health workers to reduce the behavioral risk factors of non-communicable diseases in the community: a field trial study. Arch Public Health. 2023;81(1):37.
Asadi-Aliabadi M, Tehrani-Banihashemi A, Mirbaha-Hashemi F, Janani L, Babaee E, Karimi SM, et al. Evaluating the impact of results-based motivating system on noncommunicable diseases risk factors in Iran: Study protocol for a field trial. Med J Islam Repub Iran. 2021;35:66.
PubMed PubMed Central Google Scholar
Babor TF, Higgins-Biddle JC, Higgins PS, Gassman RA, Gould BE. Training medical providers to conduct alcohol screening and brief interventions. Substance Abuse. 2004;25(1):17–26.
Baer HJ, Wee CC, Orav EJ, DeVito K, Burdick E, Williams DH, et al. Use of electronic health records for addressing overweight and obesity in rimary care: Results from a cluster-randomized controlled trial. J Gen Intern Med. 2016;31(2 SUPPL. 1):S452–3.
Bailey SR, Albert EL, Seeholzer EL, Lewis SA, Flocke SA. Sustained Effects of a Systems-Based Strategy for Tobacco Cessation Assistance. Am J Prev Med. 2023;64(3):428–32.
Balasubramanian BA, Lindner S, Marino M, Springer R, Edwards ST, McConnell KJ, et al. Improving Delivery of Cardiovascular Disease Preventive Services in Small-to-Medium Primary Care Practices. J Am Board Fam Med. 2022;35(5):968–78.
Baldeon ME, Fornasini M, Flores N, Merriam PA, Rosal M, Zevallos JC, et al. Impact of training primary care physicians in behavioral counseling to reduce cardiovascular disease risk factors in Ecuador. Rev Panam Salud Publica. 2018;42:e139.
Banerjee ES, Gambler A, Fogleman C. Adding obesity to the problem list increases the rate of providers addressing obesity. Fam Med. 2013;45(9):629–33.
PubMed Google Scholar
Bardach NS, Wang JJ, De Leon SF, Shih SC, Boscardin WJ, Goldman LE, et al. Effect of pay-for-performance incentives on quality of care in small practices with electronic health records: a randomized trial. JAMA. 2013;310(10):1051–9.
Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, Hunt JS, et al. Provider feedback to improve 5A’s tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine Tob Res. 2007;9(3):341–9.
Boston D, Larson AE, Sheppler CR, O’Connor PJ, Sperl-Hillen JM, Hauschildt J, et al. Does Clinical Decision Support Increase Appropriate Medication Prescribing for Cardiovascular Risk Reduction? J Am Board Fam Med. 2023;36(5):777–88.
Butler CC, Simpson SA, Hood K, Cohen D, Pickles T, Spanou C, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomised trial. BMJ: British Medical Journal. 2013;346(7901):10.
Campbell-Scherer DL, Asselin J, Osunlana AM, Ogunleye AA, Fielding S, Anderson R, et al. Changing provider behaviour to increase nurse visits for obesity in family practice: the 5As Team randomized controlled trial. CMAJ Open. 2019;7(2):E371.
Coleman T, Lewis S, Hubbard R, Smith C. Impact of contractual financial incentives on the ascertainment and management of smoking in primary care. Addiction. 2007;102(5):803–8.
Corelli RL, Merchant KR, Hilts KE, Kroon LA, Vatanka P, Hille BT, et al. Community pharmacy technicians’ engagement in the delivery of brief tobacco cessation interventions: Results of a randomized trial. Research in social & administrative pharmacy : RSAP. 2022;18(7):3158–63.
Cummings SR, Coater TJ, Richard RJ, Hansen B, Zahnd EG, VanderMartin R, et al. Training physicians in counseling about smoking cessation. A randomized trial of the “Quit for Life” program. Ann Intern Med. 1989;110(8):640–7.
Dubey V, Mathew R, Iglar K, Moineddin R, Glazier R. Improving preventive service delivery at adult complete health check-ups: the Preventive health Evidence-based Recommendation Form (PERFORM) cluster randomized controlled trial. BMC Fam Pract. 2006;7:44.
Evins AE, Cather C, Maravic MC, Reyering S, Pachas GN, Thorndike AN, et al. A Pragmatic Cluster-Randomized Trial of Provider Education and Community Health Worker Support for Tobacco Cessation. Psychiatr Serv. 2023;74(4):365–73.
Fichera E, Gray E, Sutton M. How do individuals’ health behaviours respond to an increase in the supply of health care? Evidence from a natural experiment. Soc Sci Med. 2016;159:170–9.
Fiore M, Adsit R, Zehner M, McCarthy D, Lundsten S, Hartlaub P, et al. An electronic health record-based interoperable eReferral system to enhance smoking Quitline treatment in primary care. Journal of the American Medical Informatics Association : JAMIA. 2019;26(8–9):778–86.
Flocke SA, Albert EL, Lewis SA, Love TE, Rose JC, Kaelber DC, et al. A cluster randomized trial evaluating a teachable moment communication process for tobacco cessation support. BMC Fam Pract. 2021;22(1):85.
Flocke SA, Seeholzer E, Lewis SA, Gill IJ, Rose JC, Albert E, et al. 12-Month Evaluation of an EHR-Supported Staff Role Change for Provision of Tobacco Cessation Care in 8 Primary Care Safety-Net Clinics. J Gen Intern Med. 2020;35(11):3234–42.
Funk M, Wutzke S, Kaner E, Anderson P, Pas L, McCormick R, et al. A multicountry controlled trial of strategies to promote dissemination and implementation of brief alcohol intervention in primary health care: findings of a World Health Organization collaborative study. J Stud Alcohol. 2005;66(3):379–88.
Girvalaki C, Papadakis S, Vardavas C, Pipe AL, Petridou E, Tsiligianni I, et al. Training General Practitioners in Evidence-Based Tobacco Treatment: An Evaluation of the Tobacco Treatment Training Network in Crete (TiTAN-Crete) Intervention. Health Educ Behav. 2018;45(6):888–97.
Goodfellow J, Agarwal S, Harrad F, Shepherd D, Morris T, Ring A, et al. Cluster randomised trial of a tailored intervention to improve the management of overweight and obesity in primary care in England. Implement Sci. 2016;11(1):77.
Grant RW, Schmittdiel JA, Neugebauer RS, Uratsu CS, Sternfeld B. Exercise as a vital sign: a quasi-experimental analysis of a health system intervention to collect patient-reported exercise levels. JGIM: Journal of General Internal Medicine. 2014;29(2):341–8.
Haller DM, Meynard A, Lefebvre D, Ukoumunne OC, Narring F, Broers B. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ : Canadian Medical Association journal. 2014;186(8):E263–72.
Harris MF, Parker SM, Litt J, van Driel M, Russell G, Mazza D, et al. An Australian general practice based strategy to improve chronic disease prevention, and its impact on patient reported outcomes: evaluation of the preventive evidence into practice cluster randomised controlled trial. BMC Health Serv Res. 2017;17:1–14.
Haskard KB, White MK, Williams SL, DiMatteo MR, Rosenthal R, Goldstein MG. Physician and patient communication training in primary care: effects on participation and satisfaction [corrected] [published erratum appears in HEALTH PSYCHOL 2009 Mar; 28(2):263]. Health Psychol. 2008;27(5):513–22.
Hilbink M, Voerman G, van Beurden I, Penninx B, Laurant M. A randomized controlled trial of a tailored primary care program to reverse excessive alcohol consumption. J Am Board Fam Med. 2012;25(5):712–22.
Houston TK, Delaughter KL, Ray MN, Gilbert GH, Allison JJ, Kiefe CI, et al. Cluster-randomized trial of a web-assisted tobacco quality improvement intervention of subsequent patient tobacco product use: a National Dental PBRN study. BMC Oral Health. 2013;13:13.
Houston TK, Sadasivam RS, Allison JJ, Ash AS, Ray MN, English TM, et al. Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implement Sci. 2015;10(1):154.
Houston TK, Sadasivam RS, Ford DE, Richman J, Ray MN, Allison JJ. The QUIT-PRIMO provider-patient Internet-delivered smoking cessation referral intervention: a cluster-randomized comparative effectiveness trial: study protocol. Implement Sci. 2010;5:87.
Hudmon KS, Corelli RL, de Moor C, Zillich AJ, Fenlon C, Miles L, et al. Outcomes of a randomized trial evaluating two approaches for promoting pharmacy-based referrals to the tobacco quitline. J Am Pharm Assoc. 2018;58(4):387–94.
Joseph AM, Nancy JA, Larry CA, Sean MN, Richard JS, Pieper CF, et al. Results of a Randomized Controlled Trial of Intervention to Implement Smoking Guidelines in Veterans Affairs Medical Centers: Increased Use of Medications without Cessation Benefit. Med Care. 2004;42(11):1100–10.
Jumbe S, Madurasinghe VW, James WY, Houlihan C, Jumbe SL, Yau T, et al. STOP- a training intervention to optimise treatment for smoking cessation in community pharmacies: cluster randomised controlled trial. BMC Med. 2022;20(1):1–14.
Kaner E, Lock C, Heather N, McNamee P, Bond S, Kaner E, et al. Promoting brief alcohol intervention by nurses in primary care: a cluster randomised controlled trial. Patient Educ Couns. 2003;51(3):277–84.
Keller S, Donner-Banzhoff N, Kaluza G, Baum E, Basler HD. Improving physician-delivered counseling in a primary care setting: lessons from a failed attempt. Educ Health (Abingdon). 2000;13(3):387–97.
Kowitt SD, Goldstein AO, Cykert S. A heart healthy intervention improved tobacco screening rates and cessation support in primary care practices. J Prev. 2022;43(3):375–86.
Lee AK, Bobb JF, Richards JE, Achtmeyer CE, Ludman E, Oliver M, et al. Integrating Alcohol-Related Prevention and Treatment Into Primary Care: A Cluster Randomized Implementation Trial. JAMA Intern Med. 2023;183(4):319–28.
Lennox AS, Bain N, Taylor RJ, McKie L, Donnan PT, Groves J. Stages of Change training for opportunistic smoking intervention by the primary health care team. Part I : randomised controlled trial of the effect of training on patient smoking outcomes and health professional behaviour as recalled by patients. Health Educ J. 1998;57(2):140–9.
Linder JA, Rigotti NA, Schneider LI, Kelley JHK, Brawarsky P, Haas JS. An electronic health record-based intervention To improve tobacco treatment in primary care a cluster-randomized controlled trial. Arch Intern Med. 2009;169(8):781–7.
Little SJ, Hollis JF, Fellows JL, Snyder JJ, Dickerson JF. Implementing a tobacco assisted referral program in dental practices. J Public Health Dent. 2009;69(3):149–55.
Malta MB, Carvalhaes MA, Takito MY, Tonete VL, Barros AJ, Parada CM, et al. Educational intervention regarding diet and physical activity for pregnant women: changes in knowledge and practices among health professionals. BMC Pregnancy Childbirth. 2016;16(1):175.
McElwaine KM, Freund M, Campbell EM, Knight J, Bowman JA, Wolfenden L, et al. Increasing preventive care by primary care nursing and allied health clinicians: a non-randomized controlled trial. Am J Prev Med. 2014;47(4):424–34.
McRobbie H, Hajek P, Feder G, Eldridge S. A cluster-randomised controlled trial of a brief training session to facilitate general practitioner referral to smoking cessation treatment. Tob Control. 2008;17(3):173–6.
Mejia R, Pérez Stable EJ, Kaplan CP, Gregorich SE, Livaudais-Toman J, Peña L, et al. EFfectiveness of an intervention to teach physicians how to assist patients to quit smoking in Argentina. Nicotine Tob Res. 2016;18(5):1101–9.
Milch CE, Edmunson JM, Beshansky JR, Griffith JL, Selker HP. Smoking cessation in primary care: a clinical effectiveness trial of two simple interventions. Prev Med. 2004;38(3):284–94.
Minian N, Baliunas D, Noormohamed A, Zawertailo L, Giesbrecht N, Hendershot CS, et al. The effect of a clinical decision support system on prompting an intervention for risky alcohol use in a primary care smoking cessation program: a cluster randomized trial. Implement Sci. 2019;14(1):85.
Minian N, Baliunas D, Zawertailo L, Noormohamed A, Giesbrecht N, Hendershot CS, et al. Combining alcohol interventions with tobacco addictions treatment in primary care-the COMBAT study: a pragmatic cluster randomized trial. Implement Sci. 2017;12(1):65.
Minian N, Lingam M, Moineddin R, Thorpe KE, Veldhuizen S, Dragonetti R, et al. The Impact of a Clinical Decision Support System for Addressing Physical Activity and Healthy Eating During Smoking Cessation Treatment: Hybrid Type I Randomized Controlled Trial. J Med Internet Res. 2022;24(9):e37900.
Moore H, Greenwood D, Gill T, Waine C, Soutter J, Adamson A. A cluster randomised trial to evaluate a nutrition training programme. Br J Gen Pract. 2003;53(489):271–7.
Moore H, Summerbell C, Vail A, Greenwood DC, Adamson AJ. The design features and practicalities of conducting a pragmatic cluster randomized trial of obesity management in primary care. Stat Med. 2001;20(3):331–40.
O’Donnell A, Angus C, Hanratty B, Hamilton FL, Petersen I, Kaner E. Impact of the introduction and withdrawal of financial incentives on the delivery of alcohol screening and brief advice in English primary health care: an interrupted time–series analysis. Addiction. 2020;115(1):49–60.
Olano-Espinosa E, Matilla-Pardo B, Minué C, Antón E, Gómez-Gascón T, Ayesta FJ. Effectiveness of a health professional training program for treatment of tobacco addiction. Nicotine Tob Res. 2013;15(10):1682–9.
Ornstein SM, Miller PM, Wessell AM, Jenkins RG, Nemeth LS, Nizetert PJ. Integration and sustainability of alcohol screening, brief intervention, and pharmacotherapy in primary care settings. J Stud Alcohol Drugs. 2013;74(4):598–604.
Patwardhan PD, Chewning BA. Effectiveness of intervention to implement tobacco cessation counseling in community chain pharmacies. Journal of the American Pharmacists Association : JAPhA. 2012;52(4):507–14.
Prokhorov AV, Hudmon KS, Marani S, Foxhall L, Ford KH, Luca NS, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Arch Intern Med. 2010;170(18):1640–6.
Ribeiro C. The family medicine approach to alcohol consumption detection and brief interventions in primary health care. Acta Medica Portuguesa. 2011;24(SUPPL.2):355–68.
Rieckmann T, Renfro S, McCarty D, Baker R, McConnell KJ. Quality Metrics and Systems Transformation: Are We Advancing Alcohol and Drug Screening in Primary Care? Health Serv Res. 2018;53(3):1702–26.
Rindal DB, Kottke TE, Jurkovich MW, Asche SE, Enstad CJ, Truitt AR, et al. Findings and Future Directions from a Smoking Cessation Trial Utilizing a Clinical Decision Support Tool. J Evid Based Dent Pract. 2022;22(3): 101747.
Rosário F, Vasiljevic M, Pas L, Angus C, Ribeiro C, Fitzgerald N. Efficacy of a theory-driven program to implement alcohol screening and brief interventions in primary health-care: a cluster randomized controlled trial. Addiction. 2022;117(6):1609–21.
Rosário F, Vasiljevic M, Pas L, Fitzgerald N, Ribeiro C. Implementing alcohol screening and brief interventions in primary health care: study protocol for a pilot cluster randomized controlled trial. Fam Pract. 2019;36(2):199–205.
Rose HL, Miller PM, Nemeth LS, Jenkins RG, Nietert PJ, Wessell AM, et al. Alcohol screening and brief counseling in a primary care hypertensive population: a quality improvement intervention. Addiction (Abingdon, England). 2008;103(8):1271–80.
Roski J, Jeddeloh R, An L, Lando H, Hannan P, Hall C, et al. The impact of financial incentives and a patient registry on preventive care quality: increasing provider adherence to evidence-based smoking cessation practice guidelines. Prev Med. 2003;36(3):291–9.
Rossom RC, Crain AL, O’Connor PJ, Waring SC, Hooker SA, Ohnsorg K, et al. Effect of Clinical Decision Support on Cardiovascular Risk Among Adults With Bipolar Disorder, Schizoaffective Disorder, or Schizophrenia: A Cluster Randomized Clinical Trial. JAMA Network Open. 2022;5(3):e220202.
Rossom RC, O’Connor PJ, Crain AL, Waring S, Ohnsorg K, Taran A, et al. Pragmatic trial design of an intervention to reduce cardiovascular risk in people with serious mental illness. Contemp Clin Trials. 2020;91:105964.
Rothemich SF, Woolf SH, Johnson RE, Burgett AE, Flores SK, Marsland DW, et al. Effect on Cessation Counseling of Documenting Smoking Status as a Routine Vital Sign: An ACORN Study. The Annals of Family Medicine. 2008;6(1):60.
Ruf D, Berner M, Kriston L, Lohmann M, Mundle G, Lorenz G, et al. Cluster-randomized controlled trial of dissemination strategies of an online quality improvement programme for alcohol-related disorders. Alcohol and alcoholism (Oxford, Oxfordshire). 2010;45(1):70–8.
Saitz R, Horton NJ, Sullivan LM, Moskowitz MA, Samet JH, Saitz R, et al. Addressing alcohol problems in primary care: a cluster randomized, controlled trial of a systems intervention. The screening and intervention in primary care (SIP) study. Ann Intern Med. 2003;138(5):372–49.
Schwartz MD, Jensen AE, Wang B, Bennett K, Dembitzer A, Strauss S, et al. The use of panel management assistants to improve smoking cessation and hypertension management by VA primary care teams: A cluster randomized controlled trial. J Gen Intern Med. 2014;29(SUPPL. 1):S234–5.
Sinclair HK, Bond CM, Lennox AS, Silcock J, Winfield AJ, Donnan PT. Training pharmacists and pharmacy assistants in the stage-of-change model of smoking cessation: a randomised controlled trial in Scotland. Tob Control. 1998;7(3):253.
Sohanpal R, Jumbe S, James WY, Steed L, Yau T, Rivas C, et al. Evaluating the effectiveness and cost-effectiveness of the Smoking Treatment Optimisation in Pharmacies (STOP) intervention: Protocol for a cluster randomised controlled trial. Trials. 2019;20(1):337.
Sturgiss E, Advocat J, Lam T, Nielsen S, Ball L, Gunatillaka N, et al. Multifaceted intervention to increase the delivery of alcohol brief interventions in primary care: a mixed-methods process analysis. Br J Gen Pract. 2023;73(735):e778–88.
Sturgiss E, Gunatillaka N, Ball L, Lam T, Nielsen S, O’Donnell R, et al. Embedding brief interventions for alcohol in general practice: a study protocol for the REACH Project feasibility trial. Bjgp Open. 2021;5(4):1–7.
Szatkowski L, Aveyard P. Provision of smoking cessation support in UK primary care: impact of the 2012 QOF revision. Br J Gen Pract. 2016;66(642):e10–5.
Twardella D, Brenner H. Effects of practitioner education, practitioner payment and reimbursement of patients’ drug costs on smoking cessation in primary care: a cluster randomised trial. Tob Control. 2007;16(1):15–21.
Unrod M, Smith M, Spring B, DePue J, Redd W, Winkel G. Randomized controlled trial of a computer-based, tailored intervention to increase smoking cessation counseling by primary care physicians. J Gen Intern Med. 2007;22(4):478–84.
van Beurden I, Anderson P, Akkermans RP, Grol RPTM, Wensing M, Laurant MGH. Involvement of general practitioners in managing alcohol problems: a randomized controlled trial of a tailored improvement programme. Addiction. 2012;107(9):1601–11.
Van Lieshout J, Huntink E, Koetsenruijter J, Wensing M. Tailored implementation of cardiovascular risk management in general practice: A cluster randomized trial. Implementation Science. 2016;11:115.
Verbiest MEA, Crone MR, Scharloo M, Chavannes NH, van der Meer V, Kaptein AA, et al. One-hour training for general practitioners in reducing the implementation gap of smoking cessation care: a cluster-randomized controlled trial. Nicotine Tob Res. 2014;16(1):1–10.
Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Ann Fam Med. 2007;5(2):135–42.
Wadlin J, Ford DE, Albert MC, Wang NY, Chander G. Implementing an EMR–Based Referral for Smoking Quitline Services with Additional Provider Education, a Cluster-Randomized Trial. J Gen Intern Med. 2022;37(10):2438–45.
Welzel FD, Bär J, Stein J, Löbner M, Pabst A, Luppa M, et al. Using a brief web-based 5A intervention to improve weight management in primary care: results of a cluster-randomized controlled trial. BMC Fam Pract. 2021;22(1):61.
Welzel FD, Stein J, Pabst A, Luppa M, Kersting A, Bluher M, et al. Five A’s counseling in weight management of obese patients in primary care: a cluster-randomized controlled trial (INTERACT). BMC Fam Pract. 2018;19(1):97.
Wiggers J, McElwaine K, Freund M, Campbell L, Bowman J, Wye P, et al. Increasing the provision of preventive care by community healthcare services: a stepped wedge implementation trial. Implement Sci. 2017;12:1–14.
Yano EM, Rubenstein LV, Farmer MM, Chernof BA, Mittman BS, Lanto AB, et al. Targeting primary care referrals to smoking cessation clinics does not improve quit rates: implementing evidence-based interventions into practice. Health Serv Res. 2008;43(5 Pt 1):1637–61.
Young JM, D’Este C, Ward JE, Young JM, D’Este C, Ward JE. Improving family physicians’ use of evidence-based smoking cessation strategies: a cluster randomization trial. Prev Med. 2002;35(6):572–83.
Baer HJ, Wee CC, DeVito K, Orav EJ, Frolkis JP, Williams DH, et al. Design of a cluster-randomized trial of electronic health record-based tools to address overweight and obesity in primary care. Clin Trials. 2015;12(4):374–83.
Coleman T. Do financial incentives for delivering health promotion counselling work? Analysis of smoking cessation activities stimulated by the quality and outcomes framework. BMC Public Health. 2010;10(1):167–72.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549.
Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C Scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcoholism: Clin Exp Res. 2013;37(8):1380–90.
Higgins-Biddle JC, Babor TF. A review of the Alcohol Use Disorders Identification Test (AUDIT), AUDIT-C, and USAUDIT for screening in the United States: Past issues and future directions. Am J Drug Alcohol Abuse. 2018;44(6):578–86.
Konnyu KJ, Yogasingam S, Lépine J, Sullivan K, Alabousi M, Edwards A, et al. Quality improvement strategies for diabetes care: Effects on outcomes for adults living with diabetes. Cochrane Database Syst Rev. 2023;5(5):Cd014513.
Tricco AC, Thomas SM, Veroniki AA, Hamid JS, Cogo E, Strifler L, et al. Quality improvement strategies to prevent falls in older adults: a systematic review and network meta-analysis. Age Ageing. 2019;48(3):337–46.
Manalili K, Lorenzetti DL, Egunsola O, O’Beirne M, Hemmelgarn B, Scott CM, et al. The effectiveness of person-centred quality improvement strategies on the management and control of hypertension in primary care: A systematic review and meta-analysis. J Eval Clin Pract. 2022;28(2):260–77.
Mandavia R, Mehta N, Schilder A, Mossialos E. Effectiveness of UK provider financial incentives on quality of care: a systematic review. The British journal of general practice : the journal of the Royal College of General Practitioners. 2017;67(664):e800–15.
Scott A, Sivey P, Ait Ouakrim D, Willenberg L, Naccarella L, Furler J, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database of Syst Rev. 2011;9:CD008451.
Knudsen SV, Laursen HVB, Johnsen SP, Bartels PD, Ehlers LH, Mainz J. Can quality improvement improve the quality of care? A systematic review of reported effects and methodological rigor in plan-do-study-act projects. BMC Health Serv Res. 2019;19(1):683.
Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2014;2014(12):Cd008743.
Wyper GMA, Mackay DF, Fraser C, Lewsey J, Robinson M, Beeston C, et al. Evaluating the impact of alcohol minimum unit pricing on deaths and hospitalisations in Scotland: a controlled interrupted time series study. Lancet. 2023;401(10385):1361–70.
Nagelhout GE, de Vries H, Boudreau C, Allwright S, McNeill A, van den Putte B, et al. Comparative impact of smoke-free legislation on smoking cessation in three European countries. Eur J Public Health. 2012;22 Suppl 1(Suppl 1):4–9.
Download references
Acknowledgements
Thanks to Corina Cheeks for her valuable PPI perspective and Nia Roberts for her search strategy expertise.
This work was supported by the Wellcome Trust [225494/Z/22/Z]. PA is an NIHR senior investigator and funded by NIHR Oxford Health Biomedical Research Centre, NIHR Oxford Biomedical Research Centre, and NIHR Oxford and Thames Valley Applied Research Collaboration.
Author information
Authors and affiliations.
Nuffield Department of Primary Care Health Sciences, University of Oxford, Radcliffe Primary Care Building, Radcliffe Observatory Quarter, Woodstock Road, Oxford, OX2 6GG, UK
Laura Heath, Richard Stevens, Brian D. Nicholson, Joseph Wherton, Min Gao, Caitriona Callan, Simona Haasova & Paul Aveyard
Department of Marketing, University of Lausanne, Quartier UNIL-Chamberonne, Lausanne, Quartier, CH-1015, Switzerland
Simona Haasova
You can also search for this author in PubMed Google Scholar
Contributions
LH, RS, BN and PA planned the study. LH, RS, PA, MG, SH and CC conducted the analysis. RS provided senior statistical support. BN, JW and PA provided supervisory support. LH wrote the initial draft. All authors commented on the final reporting of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Twitter handles
Laura Heath: @laurahheath.
Corresponding author
Correspondence to Laura Heath .
Ethics declarations
Ethics approval and consent to participate..
Not applicable.
Consent for publication.
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: database search strategy., sadditional file 2: imputed calculations., 12916_2024_3588_moesm3_esm.docx.
Additional file 3: Figures S1-S7. FigS1 – Meta-analysis summary diamonds as random effects (main analysis), Hartung-Knapp-Sidik-Jonkman (HKSJ) and inverse variance heterogeneity (IVhet) models for process outcomes. FigS2 - Meta-analysis summary diamonds as random effects (main analysis), Hartung-Knapp-Sidik-Jonkman (HKSJ) and inverse variance heterogeneity model (IVhet) models for behavioural outcomes. FigS3 - Random effects meta-analysis summary diamonds excluding studies at high risk of bias and those that required data imputation for process outcomes. FigS4 - Random effects meta-analysis summary diamonds excluding studies at high risk of bias and those that required data imputation for behavioural outcomes. FigS5 - Random effects meta-analysis of behavioural outcomes, excluding Moore 2003, Welzel 2021 and Goodfellow 2016. FigS6 - Random effects meta-analysis summary diamonds of health behaviour subgroups for process outcomes. FigS7 - Random effects meta-analysis summary diamonds of health behaviour subgroups for behavioural outcomes.
Additional file 4: Stata code.
Additional file 5: table s1 - characteristics of included studies., additional file 6: prisma 2020 for abstracts checklist., additional file 7: prisma 2020 checklist., additional file 8: table s1 - prediction interval calculation and interpretation., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Heath, L., Stevens, R., Nicholson, B.D. et al. Strategies to improve the implementation of preventive care in primary care: a systematic review and meta-analysis. BMC Med 22 , 412 (2024). https://doi.org/10.1186/s12916-024-03588-5
Download citation
Received : 18 February 2024
Accepted : 27 August 2024
Published : 27 September 2024
DOI : https://doi.org/10.1186/s12916-024-03588-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Primary care
- Implementation
- Physical activity
BMC Medicine
ISSN: 1741-7015
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Putting Evidence Into Practice: An Update on the US Preventive Services Task Force Methods for Developing Recommendations for Preventive Services
Michael j barry , md, tracy a wolff , md, mph, lori pbert , phd, karina w davidson , phd, masc, tina m fan , md, mph, alex h krist , md, mph, jennifer s lin , md, mcr, iris r mabry-hernandez , md, mph, carol m mangione , md, msph, justin mills , md, mph, douglas k owens , md, ms, wanda k nicholson , md, mph, mba.
- Author information
- Article notes
- Copyright and License information
CORRESPONDING AUTHOR : Michael J. Barry Division of General Internal Medicine Massachusetts General Hospital 100 Cambridge Street, 16 th Floor Boston, MA 02114 [email protected]
Received 2022 Jul 11; Revised 2022 Oct 24; Accepted 2022 Nov 21.
The US Preventive Services Task Force (USPSTF) is an independent body that makes evidence-based recommendations regarding preventive services to improve health for people nationwide. Here, we summarize current USPSTF methods, describe how methods are evolving to address preventive health equity, and define evidence gaps for future research.
We summarize current USPSTF methods as well as ongoing methods development.
The USPSTF prioritizes topics on the basis of disease burden, extent of new evidence, and whether the service can be provided in primary care and going forward will increasingly consider health equity. Analytic frameworks specify the key questions and linkages connecting the preventive service to health outcomes. Contextual questions provide information on natural history, current practice, health outcomes in high-risk groups, and health equity. The USPSTF assigns a level of certainty to the estimate of net benefit of a preventive service (high, moderate, or low). The magnitude of net benefit is also judged (substantial, moderate, small, or zero/negative). The USPSTF uses these assessments to assign a letter grade from A (recommend) to D (recommend against). I statements are issued when evidence is insufficient.
CONCLUSIONS
The USPSTF will continue to evolve its methods for simulation modeling and to use evidence to address conditions for which there are limited data for population groups who bear a disproportionate burden of disease. Additional pilot work is underway to better understand the relations of the social constructs of race, ethnicity, and gender with health outcomes to inform the development of a USPSTF health equity framework.
Key words: preventive medicine, clinical practice guidelines, methodology, health equity
INTRODUCTION
The US Preventive Services Task Force (USPSTF) is an independent body formed in 1984 to make evidence-based recommendations regarding preventive services including screening, behavioral counseling, and preventive drugs. The 16 members are appointed by the Director of the Agency for Healthcare Research and Quality and come from the fields of family medicine, internal medicine, nursing, obstetrics and gynecology, pediatrics, and preventive and behavioral medicine, with broad and deep expertise in preventive medicine and primary care. The USPSTF members disclose financial and nonfinancial interests. The USPSTF uses rigorous methods that include comprehensive systematic reviews addressing the benefits and harms of preventive services. The USPSTF’s recommendations are for primary care clinicians and asymptomatic patients. The objectives of this article are to describe the evolving methods of the USPSTF since prior overviews, 1 , 2 to discuss ongoing refinement of our methods and the stakeholder engagement process to address prevention more equitably, and to review our recent efforts to better classify evidence gaps. Additional details on USPSTF methods are available in its Procedure Manual. 3
Topic Nomination, Prioritization, and Updating
Recognizing the importance of diverse perspectives, any individual or group can nominate a new topic or request an update of an existing topic. The Topic Prioritization Workgroup, a subset of USPSTF members, reviews nominations to assess whether topics are in scope and focus on asymptomatic people and whether the service can be delivered or referred from primary care. The steps for topic nomination, prioritization, and updating are outlined in Figure 1 . The Workgroup also reviews active topics 2 to 3 years after publication to determine whether to keep the topic active and whether the evidence on the preventive service can be updated via an expedited process. This prioritization process is informed by a background document produced by the USPSTF’s Scientific Resource Center, providing the prevalence of the condition and relevance to primary care and summarizing new evidence and in-process studies. To better address health equity, the USPSTF now gives greater emphasis to disease burden among groups, such as Black, Hispanic/Latino, Asian and Pacific Islander, and Indigenous people, during prioritization. In addition to the background document, a prioritization survey provided by the Agency for Healthcare Research and Quality (AHRQ) and completed by USPSTF members and partner organizations informs the queue of topics for the next 12 to18 months.
Steps in topic prioritization.
TP WG = Topic Prioritization Workgroup.
Development of the Research Plan
The first step in developing or updating a recommendation is a research plan comprised of an analytic framework, key questions, and inclusion and exclusion criteria specific to the key questions and contextual questions. In the draft research plan, the USPSTF now describes steps to address equity and study heterogeneity in a new section titled Approach to Assessing Health Equity and Variation in Evidence Across Populations. The plan is developed by an Evidence-Based Practice Center (EPC) in collaboration with the USPSTF and AHRQ. The analytic framework is a graphical representation of the evidence needed to connect the performance of a preventive service to a health outcome; it depicts the population under consideration, interventions, intermediate health outcomes, and final health outcomes, capturing both benefits and harms ( Figure 2 ). Key questions articulate the chain of evidence needed to determine the net benefit of a preventive service. Contextual questions address other important considerations for the recommendation such as barriers to accessing interventions. The draft research plan is posted on the USPSTF website ( uspreventiveservicestaskforce.org ) for input.
Generic screening analytic framework.
Note: Numbers in figure correspond to key questions that are addressed by the systematic evidence review. For example, Key Question 1 relates to the direct evidence as to whether screening for a condition affects health outcomes that are important to patients. For more information about analytic frameworks, see the Task Force Procedure Manual. 3
Systematic Evidence Review
Systematic reviews addressing key questions are conducted by EPCs and follow the rigorous methods of the AHRQ EPC program 4 in addition to those of the USPSTF. These methods evolve to innovate best practices in evidence synthesis including evidence of the effect of preventive services among populations bearing a disproportionate burden of disease. The USPSTF considers randomized controlled trials and well-conducted systematic reviews and meta-analyses as methodologically strongest. Separate methods have been developed to conduct expedited reviews for topics suitable for reaffirmation. 5
Because the USPSTF has many counseling topics, recommendations include a table describing the key intervention characteristics, which allows the USPSTF to provide information to help facilitate implementation. 6 Although the systematic reviews focus on randomized controlled trials, nonrandomized studies with unbiased comparator groups may be included to address limitations in the trial evidence on the effectiveness or harms of any given preventive service. Finally, the USPSTF recognizes that improving the health of people nationwide necessitates improving the health of those who experience greater morbidity and mortality from the condition; therefore, the USPSTF continues to innovate methods to synthesize evidence for these populations 7 and integrate this evidence into recommendations. 8 , 9 This article provides additional details on our efforts to address health equity at different steps of our process.
Use of Simulation Modeling
The USPSTF commissions modeling studies 10 , 11 when empiric data are sufficient to recommend a preventive service but important questions remain. For screening, the questions are typically regarding intervals for screening, starting and stopping ages, and the screening tests used. 12 - 19 The USPSTF does not make recommendations on the basis of modeling alone without supporting empiric evidence. The USPSTF usually considers multiple models simultaneously. 11 Because these collaborative models are developed independently, they use different assumptions and structures. When collaborative models yield consistent findings, they provide a robust basis for answering remaining questions.
The recommendation statement on screening for lung cancer shows how collaborative modeling can inform important aspects of a preventive service. 18 , 19 Four independent Cancer Intervention and Surveillance Modeling Network models supplemented evidence from 2 trials showing a decrease in lung cancer mortality. These models evaluated how health outcomes varied with different start and stop ages, screening intervals, and smoking histories. 18
Recommendation Development
Assessing adequacy of evidence.
After the systematic review, the USPSTF assesses the adequacy of evidence for each key question on the basis of the body of evidence’s internal and external validity. The evidence’s adequacy to address linkage coherence across the analytic framework is also considered; that is, whether the body of evidence makes sense and fits together. The adequacy of evidence at the key question and linkage level is categorized as convincing, adequate, or inadequate. In assessing evidence adequacy, the following 6 questions are considered:
Do the studies have the appropriate research designs?
Are the studies of sufficient quality?
Are the results of the studies generalizable to the primary care population?
How many and how large are the studies?
How consistent are study results?
Are there additional factors that assist in drawing conclusions?
Assessing Magnitudes of Benefits and Harms
If the evidence is deemed convincing or adequate, the USPSTF then determines the magnitudes of benefits and harms of the preventive service. The magnitude of benefit describes the change in health outcomes that would be expected from providing vs not providing the service for a population. For example, screening interventions must lead to both earlier detection of the disease and better outcomes. Similarly, the magnitude of harm estimates the burden of harm that would be introduced by delivering the service. Given that preventive interventions are intended for asymptomatic individuals to mitigate future morbidity, the assessment of harms is critically important. The magnitude of benefit or harm is categorized as substantial, moderate, small, or zero. This estimate is based on effect sizes from studies as well as on the public health burden of the disease and the incidence, severity, and duration of outcomes. When evidence is limited, conceptual upper or lower bounds may be established by extrapolating from studies of different baseline risk populations or in settings other than primary care. Additional details are available in the USPSTF Procedure Manual. 3
Assessing Coherence Linkage
Whenever possible, the USPSTF looks for direct evidence of benefit (Key Question 1 in Figure 2 ). Direct evidence is ideal for limiting bias, providing the greatest confidence. The USPSTF also examines the indirect evidence pathway, which connects the target population (far left of Figure 2 ) to improved health outcomes (far right of Figure 2 ) by linking Key Questions 3 to 8 (How accurate are screening tools? How well does treatment work? Can intermediate outcomes predict health outcomes? What are the harms of each step?). To make this linkage, the USPSTF looks at the coherence of the evidence, or how well the pieces fit together, and the applicability of the evidence to an asymptomatic primary care population. Compared to direct evidence, indirect evidence has a greater risk of bias.
Intermediate Outcomes
The Task Force defines a health outcome as a symptom, functional level, or condition that patients can feel or experience. Examples include functional status, quality of life, and mortality. Available studies on preventive services often do not report on health outcomes but instead on intermediate outcomes ( Figure 2 ). Intermediate outcomes are pathologic, physiologic, social, or behavioral measures that a patient does not feel or experience. A preventive service might affect an intermediate outcome without improving health outcomes. The USPSTF has developed methods for considering the linkage between intermediate and health outcomes. 20 When assessing linkage, the USPSTF looks for evidence showing a consistent relation between a change in an intermediate outcome and a change in health outcome. For example, regarding hepatitis C screening ( Supplemental Figure 1 ), there was convincing evidence that newer antiviral regimens resulted in a sustained virologic response (an intermediate outcome) in a very high proportion of adults and adequate evidence of a consistent association between sustained virologic response and improved health outcomes (decreased all-cause and liver disease mortality). Given this linkage, the USPSTF issued a B recommendation for hepatitis C virus screening. 21 , 22
Determining a Recommendation Grade
To make a recommendation, the USPSTF judges the certainty and magnitude of the net benefit (benefits minus harms) of the preventive service at the population level. Certainty, categorized as high, moderate, or low, is based on the quality of the evidence (see USPSTF Procedure Manual 3 for more detail). Assessing certainty requires a synthesis of evidence across the analytic framework to judge whether the results observed would be expected when the intervention is delivered for primary care populations and how likely future research would change that assessment.
The magnitude of net benefit is categorized as substantial, moderate, small, zero, or negative. Assessing net benefit can be challenging because the metrics for benefits and harms often differ. For example, screening for prostate cancer with the prostate-specific antigen (PSA) test among men aged 55-69 years might prevent approximately 1 death per 1,000 men screened over a period of 10 years. Yet screening leads to many more men having a false-positive result and receiving a diagnosis of prostate cancer, leading to overdiagnosis and often overtreatment of cancers not destined to cause harm ( Figure 3 ). 23 , 24 Ultimately after extensive discussion, the USPSTF judged with moderate certainty that PSA screening provided a small net benefit. In addition, there might be insufficient evidence on net benefit to support an alternative screening strategy for populations at greater risk of the disease. For example, Black men have a lifetime probability of dying of prostate cancer of approximately 5% compared with 3% for White men. However, available evidence did not support a different PSA screening recommendation for Black men in part because of a lack of sufficient inclusion of Black men in the reviewed evidence. A call for more research was highlighted in the recommendation.
Estimates of the benefits and harms of PSA screening for prostate cancer.
PSA = prostate-specific antigen.
Note: Reprinted with permission from the Agency for Healthcare Research and Quality, acting on its own behalf and that of the US Preventive Services Task Force.
Table 1 shows how a letter grade is determined for a preventive service using the USPSTF estimates of certainty and net benefit. If certainty is low, an Insufficient Evidence or I statement is issued; the USPSTF does not use expert opinion to make recommendations when evidence is lacking. 25 , 26
United States Preventive Services Task Force Recommendation Grade Grid: Magnitude and Certainty of Net Benefit
Communicating Recommendations
In its statements, the USPSTF describes the chain of evidence used to arrive at the recommendation in the Assessment of Magnitude of Net Benefit section and the Rationale Table. The evidence informing the recommendation is summarized in the Supporting Evidence section.

Understanding Grades
Table 2 provides a definition of each letter grade and corresponding suggestions for practice. For example, it was determined with moderate certainty that screening for hepatitis C virus infection in a population aged 18 to 79 years has substantial net benefit; therefore, a B grade was issued, meaning that clinicians should routinely recommend screening. 21
How to Interpret Task Force Recommendation Grades
USPSTF = United States Preventive Services Task Force.
Practice Considerations
The Practice Considerations section provides clinicians a concise, streamlined summary of information needed to implement the recommendation. 27 Companion materials may include infographics and office conversation guides.
Research Gaps
The USPSTF includes a Research Needs and Gaps section in its recommendations to communicate key research still needed. 27 The USPSTF has become increasingly concerned about widespread inequities in preventive care such as those based on sex, gender, race, and ethnicity. The USPSTF is especially attuned to inequities in Black, Hispanic/Latino, Asian and Pacific Islander, and Indigenous populations that face systemic racism leading to greater risks of preventable diseases and a lower likelihood of receiving appropriate preventive services followed by diagnosis and treatment. 8 , 9 The USPSTF continues to report research gaps addressing health inequities to the US Congress and research funders.
Stakeholder Engagement
The USPSTF values input from the public, specialists, and other stakeholders at every stage of the recommendation process. Via the USPSTF website ( uspreventiveservicestaskforce.org ), anyone can nominate topics and provide feedback on draft research plans, recommendation statements, and evidence reports. Every comment is considered by USPSTF members before finalization of documents. In addition, the USPSTF reaches out to stakeholder organizations directly and invites them to provide comments. All draft evidence reports are reviewed by experts in the field and USPSTF federal health partners; organizations with content expertise are also invited to nominate reviewers. The USPSTF continuously engages with federal and nonfederal partners via regular meetings. This feedback makes recommendations more understandable to clinicians and stakeholders.
Ongoing Methods Development
The USPSTF is dedicated to meeting the health needs of an increasingly diverse US population and recognizes the impact of social determinants on the delivery of preventive services. Given that many important population groups, particularly groups bearing a disproportionate burden of disease, are often not included in trials, the USPSTF continues to refine its methods to develop evidentiary rules (for nonrandomized studies, epidemiologic data, and modeling) and criteria for extrapolation to better address racial, ethnic, and gender disparities in the use of preventive services and in health outcomes.
The approach to addressing inequities is exemplified in the recent update of the USPSTF lung cancer screening recommendation. 19 The updated recommendation was informed by new trial evidence and simulation modeling 18 that allowed the USPSTF to identify the most efficient screening strategies, particularly among Black people, who have a greater burden of lung cancer. On the basis of simulation modeling, the 2021 recommendation, which decreased the starting age from 55 to 50 years and the smoking criterion from ≥30 to ≥20 pack-years, would increase the relative percentage of adults eligible for screening by 78% in non-Hispanic White persons, 107% in non-Hispanic Black persons, and 112% in Hispanic/Latino persons.
Potential approaches under consideration to address equity are the use of robust comparative cohort or interrupted time series studies with sufficient participant diversity to identify variations in net benefits by race, ethnicity, sex, gender, or social determinants of health. Additional analytic approaches, such as individual participant meta-analyses and modeling with race as an independent variable, may also be considered. Sex and gender of participants are not often clearly specified in studies of preventive services. The USPSTF is developing inclusive approaches to addressing sex and gender in recommendation development. 28 Additional approaches include a taxonomy to categorize evidence gaps and inform future research addressing health inequities. 29 As these changes crystalize, they will be reflected in updates to the Task Force Procedure Manual. 3
There is a need to assess whether these approaches decrease any influence of systemic racism or sources of bias and inequity at each step of recommendation formation; for example, whether recommendations might create implementation barriers that disproportionately affect some population groups. This process will also inform the development of a health equity framework that aligns with these approaches. As part of future evidence reviews and as outlined in prior articles, 8 , 9 the USPSTF will continue to pilot test the inclusion of evidence on variation in benefits and harms as well as implementation barriers by population groups.
The USPSTF is committed to recommending evidence-based clinical preventive services. Achieving this goal is critical to the health of a diverse US population. The USPSTF will continue to follow its traditional robust critical appraisal of the evidence while working to advance innovative methods to address conditions for which there are limited data for specific disproportionately affected groups. This evolution of methods will ensure that the USPSTF meets its mission of improving the health of all people nationwide.
Supplementary Material
Conflicts of interest: authors report none.
Read or post commentaries in response to this article.
Disclaimer: Drs Krist, Mangione, Owens, Davidson, Pbert, Barry, and Nicholson are current or recent members of the independent US Preventive Services Task Force (USPSTF), and they speak ex cathedra for the Task Force. Drs Mabry-Hernandez, Mills, Fan, and Wolff are staff at the Agency for Healthcare Research and Quality (AHRQ), and Dr Lin works with the USPSTF via a contract with the AHRQ. The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the AHRQ. No statement in this manuscript should be taken as an official position of the AHRQ or the US Department of Health and Human Services.
Supplemental materials
- 1. Harris RP, Helfand M, Woolf SH, et al. ; Methods Work Group, Third US Preventive Services Task Force . Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001; 20(3 Suppl): 21-35. 10.1016/s0749-3797(01)00261-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Krist AH, Barry MJ, Wolff TA, Owens DK, Fan TM, Davidson KW.. Evolution of the U.S. Preventive Services Task Force’s methods. Am J Prev Med. 2020; 58(3): 332-335. 10.1016/j.amepre.2019.11.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. US Preventive Services Task Force . Procedure Manual. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual . Accessed Nov 24, 2021.
- 4. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Content last reviewed January 2020. Effective Health Care Program, Agency for Healthcare Research and Quality. https://effectivehealthcare.ahrq.gov/products/collections/cer-methods-guide . Accessed Jan 2, 2023. [ Google Scholar ]
- 5. Patnode CD, Eder ML, Walsh ES, Viswanathan M, Lin JS.. The use of rapid review methods for the U.S. Preventive Services Task Force. Am J Prev Med. 2018; 54(1S1): S19-S25. 10.1016/j.amepre.2017.07.024 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014; 348: g1687. 10.1136/bmj.g1687 [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Bibbins-Domingo K, Whitlock E, Wolff T, et al. Developing recommendations for evidence-based clinical preventive services for diverse populations: methods of the U.S. Preventive Services Task Force. Ann Intern Med. 2017; 166(8): 565-571. 10.7326/M16-2656 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Doubeni CA, Simon M, Krist AH.. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA. 2021; 325(7): 627-628. 10.1001/jama.2020.26188 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. US Preventive Services Task Force; Davidson KW, Mangione CM, Barry MJ, et al. Actions to transform US Preventive Services Task Force methods to mitigate systemic racism in clinical preventive services. JAMA. 2021; 326(23): 2405-2411. 10.1001/jama.2021.17594 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Owens DK, Whitlock EP, Henderson J, et al. ; US Preventive Services Task Force . Use of decision models in the development of evidence-based clinical preventive services recommendations: methods of the U.S. Preventive Services Task Force. Ann Intern Med. 2016; 165(7): 501-508. 10.7326/M15-2531 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Petitti DB, Lin JS, Owens DK, Croswell JM, Feuer EJ.. Collaborative modeling: experience of the U.S. Preventive Services Task Force. Am J Prev Med. 2018; 54(1S1): S53-S62. 10.1016/j.amepre.2017.07.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal cancer screening: an updated modeling study for the US Preventive Services Task Force. JAMA. 2021; 325(19): 1998-2011. 10.1001/jama.2021.5746 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021; 325(19): 1965-1977. 10.1001/jama.2021.6238. Erratum in: JAMA . 2021; 326(8): 773. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Kim JJ, Burger EA, Regan C, Sy S.. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018; 320(7): 706-714. 10.1001/jama.2017.19872 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018; 320(7): 674-686. 10.1001/jama.2018.10897 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med. 2016; 164(4): 215-225. 10.7326/M15-1536 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Siu AL; US Preventive Services Task Force . Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016; 164(4): 279-296. 10.7326/M15-2886. Erratum in: Ann Intern Med . 2016; 164(6): 448. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: a Collaborative Modeling Study for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2021. Report No. 20-05266-EF-2. Accessed Jan 2 2023. ncbi.nlm.nih.gov/books/NBK568586 [ PubMed ] [ Google Scholar ]
- 19. US Preventive Services Task Force; Krist AH, Davidson KW, Mangione CM, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021; 325(10): 962-970. 10.1001/jama.2021.1117 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Wolff TA, Krist AH, LeFevre M, et al. Update on the methods of the U.S. Preventive Services Task Force: linking intermediate outcomes and health outcomes in prevention. Am J Prev Med. 2018; 54(1S1): S4-S10. 10.1016/j.amepre.2017.08.032 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020; 323(10): 970-975. 10.1001/jama.2020.1123 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Chou R, Dana T, Fu R, et al. Screening for hepatitis C virus infection in adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020; 323(10): 976-991. 10.1001/jama.2019.20788. Erratum in: JAMA . 2020; 323(13): 1318. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018; 319(18): 1901-1913. 10.1001/jama.2018.3710. Erratum in: JAMA . 2018; 319(23): 2443. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J.. Prostate-specific antigen-based screening for prostate cancer: a systematic evidence review for the US Preventive Services Task Force. JAMA. 2018; 319(18): 1914-1931. 10.1001/jama.2018.3712 [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Yao L, Ahmed MM, Guyatt GH, et al. Discordant and inappropriate discordant recommendations in consensus and evidence based guidelines: empirical analysis. BMJ. 2021; 375: e066045. 10.1136/bmj-2021-066045 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Yao L, Guyatt GH, Djulbegovic B.. Can we trust strong recommendations based on low quality evidence? BMJ. 2021; 375: n2833. 10.1136/bmj.n2833 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Epling JW, Borsky AE, Gerteis J.. Improvements to the US Preventive Services Task Force Recommendation Statement. JAMA. 2019; 322(12): 1143-1144. 10.1001/jama.2019.11311 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Caughey AB, Krist AH, Wolff TA, et al. USPSTF approach to addressing sex and gender when making recommendations for clinical preventive services. JAMA. 2021; 326(19): 1953-1961. 10.1001/jama.2021.15731 Erratum in: JAMA . 2021; 326(23): 2437. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. National Academies of Sciences, Engineering, and Medicine. Wojtowicz A, Stratton K, Lieu TA, Eds. Closing evidence gaps in clinical prevention. National Academies Press; 2022. 10.17226/26351 [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (642.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Open access
- Published: 22 June 2024
BETTER LIFE- guidelines for chronic disease preventive care for people aged 18–39 years: a literature review
- Nasheed Moqueet 1 ,
- Sylvie D. Cornacchi 2 ,
- Jesmin Antony 3 ,
- Ielaf Khalil 4 ,
- Donna Manca 5 ,
- Carolina Fernandes 5 ,
- Lawrence Paszat 6 ,
- Kris Aubrey-Bassler 7 ,
- Eva Grunfeld 8 , 13 ,
- Nicolette Sopcak 5 ,
- Andrew Pinto 9 ,
- Jill Konkin 5 ,
- Candace Nykiforuk 10 ,
- Linda Rabeneck 11 ,
- Peter Selby 12 , 13 ,
- Becky Wall 14 ,
- Mary Ann O’Brien 13 na1 &
- Aisha Lofters 3 , 13 na1
BMC Primary Care volume 25 , Article number: 224 ( 2024 ) Cite this article
1390 Accesses
1 Citations
11 Altmetric
Metrics details
The original ‘BETTER’ (Building on Existing Tools To Improve Chronic Disease Prevention and Screening in Primary Care) approach consisted of a prevention-focused visit between participants aged 40–65 years and a “Prevention Practitioner” (PP), who empowered the participant to set achievable prevention and screening goals for cancers and chronic diseases. BETTER was successfully adapted for economically deprived communities (BETTER HEALTH) in Canada. Our objective was to conduct a review of guidelines in preparation for adapting the ‘BETTER HEALTH’ approach for younger adults aged 18–39 years living with lower income, a group known to have earlier mortality due to a higher prevalence of preventable chronic diseases than their peers with higher income.
We searched multiple electronic databases and grey literature for clinical practice guidelines on prevention/screening and included those that met the following criteria: published in English from 2008–2020 in Canada or any of the following countries (Australia, Ireland, New Zealand, Scotland, United States and England); and addressed prevention or screening. We assessed quality using the Appraisal of Guidelines for Research and Evaluation (AGREE) II tool and extracted data (publication details, recommendations, and Quality/Level of evidence as reported by authors) from sources with overall scores of 5 or higher. Final recommendations were compiled after harmonization with input from diverse stakeholders (co-investigators, PPs, and the Community Advisory Committee).
We included a total of 85 guidelines, and developed a final list of 42 recommendations for 18–39 year-olds across 21 topics. Specific recommendations fell under the following topics: cancers, cardiovascular disease, diabetes, obesity, lifestyle (alcohol; healthy nutrition/physical activity); healthy relationships and healthy sexuality, immunization, oral health, social determinants of health, and substance use .
We identified evidence-based guidelines on individual-level prevention/screening actions for adults 18–39 years old and relevant for those living with lower income which will directly inform development and implementation of the BETTER LIFE intervention.
Peer Review reports
Introduction
Despite the existence of strong evidence for lifestyle modifications and for screening and preventive actions to improve health outcomes, an implementation gap exists due to limited physician time [ 1 ], conflicting/unclear guidelines, and difficulties inherent to sustained behaviour change [ 2 ]. The original BETTER (Building on Existing Tools To Improve Chronic Disease Prevention and Screening in Primary Care) intervention was designed to address this gap by providing an integrated approach to increasing uptake of chronic disease prevention and screening (CDPS) actions using a framework of shared decision-making between patient and practitioner. In a pragmatic cluster randomised control trial (RCT), the BETTER approach improved the uptake of CDPS actions against heart disease, diabetes and several cancers (colorectal, breast and cervical cancers) by 32.5% in urban primary care settings in Alberta and Ontario, Canada [ 2 , 3 ]. The intervention consisted of an individual prevention-focussed visit between participants aged 40–65 years and a “Prevention Practitioner” (PP), who used principles of motivational interviewing to empower the participant to set achievable prevention and screening goals, based on the harmonization of evidence, which were then recorded in a goals sheet and a personalized ‘prevention prescription’.
There have been subsequent modifications of the BETTER approach with similar positive results. ‘BETTER 2’ targeted the same age group as the original BETTER but modified the approach for different populations due to equity concerns, including individuals from rural, lower income, or historically marginalized backgrounds in Newfoundland and Labrador and the Northwest Territories, Canada [ 4 ]. Subsequently, BETTER WISE (Building on Existing Tools to Improve Cancer and Chronic Disease Prevention and Screening in Primary Care for Wellness of Cancer Survivors and Patients) tailored the BETTER approach for cancer survivors (breast, colorectal, prostate) aged 40–65 and also included screening for poverty, as well as an updated literature review to recommend specific prevention and screening actions [ 5 ]. Another modified version, BETTER HEALTH: Durham used a public health-led model with public health nurses serving as PPs for 40–64 year-olds living with lower income in Durham, Ontario, and found a 53% increase in completed health actions (immediate intervention, n = 60 vs. wait-listed arm, n = 66) [ 6 , 7 ]. Although there were positive results for this age group, the community advisory group for BETTER HEALTH: Durham suggested that starting the intervention at 40 years of age was too late for people living with low income, where evidence shows an earlier onset of chronic diseases [ 8 ]. We aimed to adapt the BETTER HEALTH: Durham intervention to a new population of adults aged 18–39 years living with low income, a group known to have earlier mortality due to, and higher prevalence of, preventable chronic diseases than their peers with higher income.
To support the adaptation, we conducted a review of guidelines to identify and assess prevention and screening actions for health issues and risk factors amenable to individual change for the 18–39 year age group. This paper describes the methods and results of the literature review.
Overview of search strategy
First, we assessed the data sources (clinical practice guidelines) from the most recent BETTER WISE study [ 9 ], which had entailed a rigorous evidence review process to recommend specific prevention and screening actions, for applicability to adults aged 18–39 years.Then, we used a structured grey literature search of specific repositories and websites to find relevant clinical practice guidelines for new topics suggested by the research team. If guidelines were unavailable for these topics, we performed a systematic literature search in the databases Ovid Medline, CINAHL (Cumulated Index to Nursing and Allied Health Literature), and the Cochrane Database of Systematic Reviews to identify systematic reviews/meta-analyses. Thus, our search and eligibility criteria for new sources was restricted to clinical practice guidelines (i.e. excluding systematic reviews, meta-analyses, and review of reviews when guidelines were found) and expanded to allow systematic reviews and meta-analyses when guidelines were not available (See Fig. 1 ).

Search strategy for guidelines for BETTER LIFE
Search strategy for topics of interest
To create the overall search strategy, we consulted an experienced information specialist (CZ). We used different combinations of key words such as ‘guidelines’, ‘chronic disease prevention’, ‘prevention’, ‘clinical practice guidelines’, and ‘screening’ with terms from topics of interest from previous versions of BETTER ( cardiovascular disease, diabetes, cancer, obesity, diet and nutrition, physical activity, smoking/tobacco and alcohol use) and new topics suggested by the wider research team (co-investigators, PPs, Community Advisory Committee (CAC)) due to their importance for our target population (See Supp Table 1 ).
Search sources
We conducted a structured search in repositories of guidelines at the provincial level (Ontario, Alberta, Newfoundland & Labrador): Cancer Care Ontario; Cancer Control Alberta; Eastern Health Cancer Care Program; and national level: Health Canada; Public Health Agency of Canada (PHAC); and the Canadian Task Force on Preventive Health Care (CTFPHC). (Details in Fig. 1 ).
We did not find guidelines for four topics recommended by our study team for our target population ( speeding, texting & driving, seat belts, bullying & cyberbullying). Therefore, we then conducted a systematic search on select databases (Ovid Medline, CINAHL, Cochrane Database of Systematic Reviews) for systematic reviews and meta-analyses published from 2008-August 2020 on these topics.
Inclusion & exclusion criteria
When screening abstracts obtained from our searches, we included articles for full-text review if they met the following criteria: clinical practice guidelines in English only; published from 2008–2020; country of publication was Australia, Canada, England, Ireland, New Zealand, Scotland, or US; included at least one of the identified topics in title or abstract; and addressed prevention or screening.
At full-text screening, we excluded articles if they met any of the following: exclusively focused on management or treatment; exclusively targeted ages not 18–39 years old (i.e., under 18, 40 or older); lacked individual-level recommendations (i.e. contained only macro-level data (e.g. legal, policy)); or lacked evidence of synthesis. With the exception of the four topics covered during the systematic search, we also excluded full-texts if they were systematic reviews, review of reviews, or meta-analyses.
During full-text screening, if multiple eligible sources existed, we used a hierarchical approach to determine inclusion: preference for most recent Canadian guideline/ review and if not available, relevant guidelines from any of 6 aforementioned primarily English-speaking countries of interest. If there were discrepancies or disagreements among guidelines, we searched for and extracted information from primary or common references.
All abstracts and full-texts were uploaded and screened using Covidence [ 10 ].
Quality assessment
We chose AGREE-II for quality assessment since it was developed specifically for assessing quality of existing practice guidelines, unlike GRADE (Grading of Recommendations, Assessment, Development, and Evaluations), which is most suited for developing guidelines de novo and for rating primary sources of evidence for specific outcomes, which was outside the scope of our study. We used a two-step process to assess guideline quality. For the first step, two trained reviewers (NM and SC) independently used a shorter 2-item AGREE-II [ 11 ] rating system to assess the “Rigour of development” (items 7 and 12—‘ Systematic methods were used to search for evidence’ and ‘There is an explicit link between the recommendations and the supporting evidence’ , respectively) on all references. If methodological details were missing from guidelines, we emailed authors or guideline developers to request more information. Both reviewers had to assign a score of 4 or higher (out of 7) on both AGREE-II items for the article to move to full quality assessment with the 23-item AGREE-II tool.
Specifically, the reviewers examined the ‘ Methods ’ section of each guideline to assess the details of systematic methods (Item #7) that were used and consulted any methods papers that governed the overall initiative when available [ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]. If the guideline developer did not report any evidence of an independent synthesis as per the first step in the AGREE–II screening process, the guideline was not assessed further. If no Canadian reference met the criteria for the 2-item AGREE-II screening tool on a given topic, the reviewers then assessed the quality of the non-Canadian documents. Disagreement over scores was discussed and a final decision was determined by consensus.
For step 2, two reviewers independently applied the full AGREE-II instrument on all guidelines that passed the 2-item screening. Overall scores of 5 and above (out of 7) by both reviewers were used to move to full data extraction phase. To ensure consistent interpretation of data quality, we pilot tested the full AGREE-II tool on 5 articles that had previously been included in BETTER WISE and that also met the eligibility criteria for BETTER LIFE.
Data extraction
Two reviewers also pilot tested the data extraction form on 5 articles and resolved differences by consensus. Each reviewer independently extracted data from half the included articles and then checked a subset from the other reviewer for consistency, resolving differences by discussion. Extracted data included publication details (issuing body/author, year and country of publication), participant characteristics (target population, age, ethnicity, socioeconomic metrics, identified risk factors, clinical context) and guideline details (individual-level recommendations, quality of supporting evidence, and whether conflict of interest was declared or not).
Harmonization and synthesis of extracted data
The extracted data were grouped by topics. Each article was assigned to two reviewers who independently either categorized recommendations for inclusion in BETTER LIFE or excluded them if they were duplicative, out of scope, or not actionable (See Fig. 2 ).

Harmonization process for BETTER LIFE
The reviewers met to discuss and assign a final primary categorization to each recommendation with the overall team meeting to resolve differences if there was no agreement between reviewers. The senior co-authors (AL and MAO) reviewed the categorizations, clarified unclear recommendations and identified specific recommendations for further review from content experts/co-investigators in the BETTER team.
Harmonization and synthesis
We followed a similar harmonization process to Campbell-Sherer et al [ 9 ] within an overarching ADAPT-ITT framework [ 21 ].
All the co-investigators and PPs in the BETTER team were invited to provide input on topics in which they had expertise and asked to rank the newly included recommendations in an online survey (Qualtrics, Provo, UT), with the goal of reaching consensus on the top ranked (most relevant) recommendations. Recommendations ranked with a mean of 90% or above were included, while those that were that consistently ranked low (mean of less than 75%) were removed. For topics with multiple individual recommendations with mean scores of 80–89%, we combined, summarized and simplified the multiple recommendations where it seemed appropriate to do so and included them.
After the harmonization process, we compiled the final list of recommendations and topics into a table and also grouped all related included topics into existing or new ‘domains’ for data visualization.
There were 864 abstracts, of which 762 were unique. Of these, 435 were moved to the full-text phase and assessed for inclusion. One hundred and eighty-five guidelines met the inclusion criteria for quality assessment (Fig. 3 a).

a Summary flow from literature search to full-text review for quality assessment. b Quality assessment of guidelines using the AGREE-II instrument to the data extraction stage
From the 150 guidelines included in BETTER WISE that were published in 2008 or later, 40 guidelines were applicable to the 18–39 year age group, of which 14 had been updated since inclusion in BETTER WISE. Newer versions were available for the following 8 topics: cancers (breast, cervical, colorectal), CVD, diabetes, obesity, lifestyle (alcohol; healthy nutrition/physical activity) .
From the search for topics for which there were no identified guidelines (speeding, texting and driving, seat belts, bullying and cyberbullying) , 213 papers were uploaded into Covidence after removing duplicates. However, all the papers on these topics were excluded at various stages.
One hundred and eighty-five guidelines were eligible for quality screening (Fig. 3 b). After exclusion at various stages, 93 guidelines were rated with the 2-item AGREE-II. Of these, 75 were rated with the full AGREE-II tool and 58 papers (77%, 58/75) were scored 5 or higher by both reviewers.
We extracted data from 85 guidelines (58 were new guidelines and 27 were from previous versions of BETTER). Of the 38 new topics (Supp Table 1 ), 22 were relevant to the 18–39 year age group (Supp Table 2).
Of the 19 colleagues invited, 9 responded, reporting expertise on atleast one of the topics on the list (between 1–8 respondents provided ranking on each of the various new recommendations). At the harmonization stage, the team removed the topic ‘ falls/injury prevention’ as the recommendation was deemed not in scope for the 18–39 age group.
Due to low ranking scores from Co-investigators, we removed 6 topics from inclusion in the final BETTER LIFE recommendations ( intimate partner violence, sexual health, skin cancer, sleep, violence, vitamins ). We also excluded hepatitis C as only one co-investigator provided a ranking for this recommendation, and the recommendation was to not screen for hepatitis C. On the advice of the research team, we also included screening for Adverse Childhood Experiences (ACE) [ 22 , 23 ].
Based on the results of the data extraction and harmonization, the final list of topics contained 42 recommendations for 18–39 year-olds across 21 total topics (Table 1 ). We grouped the final list of topics into existing or new domains (See Supp Fig. 1 ).
The CDPS recommendations for heart disease and colorectal and breast cancers were only targeted to those deemed ‘high-risk’ (based on various clinical criteria such as family history) in the 18–39 age group. For most of the new topics, we also identified specific maneuvers or screening questions/tools that could be incorporated into the BETTER visits or into BETTER tools.
We used a structured search of published and grey literature, and a systematic search of specific databases to compile recent evidence from clinical practice guidelines on risk factors and individual prevention and screening actions relevant to adults aged 18–39 years, particularly those living with low income, in Canada. We also obtained input from our co-investigators, a team of experts in primary care, public health, the social determinants of health, and the BETTER program. Through this process, we were able to identify 42 recommendations within 21 total topics that will be applied in the BETTER LIFE approach for younger adults living with low income.
Some topics and health recommendations from previous BETTER versions were updated or included, such as those addressing diabetes, cardiovascular disease, cancer, smoking, alcohol, nutrition, and exercise . Risk assessments for diabetes, cardiovascular disease and most cancers were similar for those aged 18–39 years old as with previous versions of BETTER, though routine screening was only recommended for those deemed high risk (with the exception of cervical cancer screening). We found evidence-based guidelines addressing new topics relevant specifically to 18–39 year olds grouped into the following new domains: healthy relationships and healthy sexuality, immunization, oral health, social determinants of health, and substance use . Some recommendations in BETTER LIFE were similar to those published by others [ 97 , 98 , 99 ], though the recency, diversity, and sources of our search; our harmonization and implementation process, as well as the definition of our target population were different. For example, Persaud et. al. developed 15 preventive care recommendations and 1 policy recommendation that promote health equity in Canada. Although their work and ours both prioritize health equity in primary care, Persaud et al. did not have any age restrictions on their target population nor a primary focus on uptake of individual-level preventive actions. They also utilized systematic reviews, primary research articles and randomized controlled trials to develop recommendations using a GRADE approach. Because we prioritized recommendations that were individually actionable, supported by evidence that met our criteria, and ranked highly by content experts, topics like vitamins and skin cancer prevention were eventually omitted. Although we ultimately excluded skin cancer , this topic is an important one in many countries such as Australia [ 100 ].
Taking specific contexts into account is important when determining how best to implement and support uptake of the recommendations. For some new topics, we found stronger evidence for resources and screening tools for PPs than for specific recommendations (e.g. the National Institute on Drug Abuse (NIDA) Quick Screen or the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) for substance use ). PPs identified local community resources for some new health topics ( parenting; substance use; oral health ) which could help to support participants achieve recommended actions. They also suggested considering social contexts as opportunities for engagement, e.g. by focusing conversations in BETTER LIFE visits on the concepts of health promotion or meaningful overall health and social well-being rather than explicit chronic disease prevention; by using different media for sharing health information (e.g. mobile apps, social media or online resources); by considering social contexts as barriers or enablers of behaviour change, especially regarding physical activity, alcohol, substance use ; or by taking life stage into account (single adult vs. parenting).
Our study had several strengths and limitations. Our strengths include a rigorous critical appraisal of the literature with a two-step quality assessment process and independent review that ensured that only guidelines that met high methodological rigour and transparency were included for data extraction and harmonization; focus on actionable recommendations (e.g. goal-setting, access/referral to community resources); and meaningful collaborations with diverse community, public health, and clinical experts. However, all the guidelines were published prior to the COVID-19 pandemic, so did not take pandemic-related disruptions and health impact into account. COVID-19 has exacerbated health and economic inequities and disproportionately affected racialized and low income groups with a higher risk of exposure due to living and working conditions; higher prevalence of co-morbidities; inequitable access to testing and treatment; and disruption of health services [ 101 , 102 ]. We also relied on consensus to resolve disagreements during the screening process and to formulate the final recommendations as well as on voluntary responses during harmonization which led to varied numbers of reviewers for each recommendation, and which may be subject to bias. However, we used AGREE-II to ensure transparency and careful documentation, and also consulted a wide and diverse range of experts (in primary care, public health, the social determinants of health, Prevention Practitioners, and the Community Advisory Committee) at many stages of the project. Finally, we may have missed guidelines because we targeted our search to specific criteria, repositories, and databases.
Adults living with low income are at increased risk of chronic disease. Through critical literature review and guideline harmonization, we have curated a list of individual-level actionable recommendations relevant to prevention and screening for people aged 18–39 living with low income in English-speaking countries.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
Adverse Childhood Experiences
Assessment, Decision, Adaptation, Production, Topical Experts, Integration, Training, Testing
Appraisal of Guidelines for Research & Evaluation II
Alcohol, Smoking and Substance Involvement Screening Test
Alcohol Use Disorders Identification Test
Building on Existing Tools To Improve Chronic Disease Prevention and Screening in Primary Care
Building on Existing Tools to Improve Cancer and Chronic Disease Prevention and Screening in Primary Care for Wellness of Cancer Survivors and Patients
Body mass index
Blood pressure
Community Advisory Committee
Chronic disease prevention and screening
Cumulated Index to Nursing and Allied Health Literature
Canadian Task Force on Preventive Health Care
Coronavirus disease 2019
Cardiovascular disease
Estimated glomerular filtration rate
Electronic nicotine delivery systems
Generalized Anxiety Disorder 2-item
Human papillomavirus
Manual office blood pressure device
National Institute on Drug Abuse
Public Health Agency of Canada
Primary care provider
Prevention Practitioner
Randomised control trial
Sexually transmitted infection
United States
Yarnall KSH, Pollak KI, Østbye T, Krause KM, Michener JL. Primary Care: Is There Enough Time for Prevention? Am J Public Health. 2003;93(4):635–41. https://doi.org/10.2105/AJPH.93.4.635 .
Article PubMed PubMed Central Google Scholar
Grunfeld E, Manca D, Moineddin R, et al. Improving chronic disease prevention and screening in primary care: results of the BETTER pragmatic cluster randomized controlled trial. BMC Fam Pract. 2013;14(1):175. https://doi.org/10.1186/1471-2296-14-175 .
BETTER. BETTER website. http://better-program.ca/evidence/ . Accessed Jan 23, 2022.
Aubrey-Bassler K, Fernandes C, Penney C, et al. The effectiveness of a proven chronic disease prevention and screening intervention in diverse and remote primary care settings: an implementation study on the BETTER 2 Program. BJGP Open. 2019;3(3):bjgpopen19X101656 https://doi.org/10.3399/bjgpopen19X101656 .
Manca DP, Fernandes C, Grunfeld E, et al. The BETTER WISE protocol: building on existing tools to improve cancer and chronic disease prevention and screening in primary care for wellness of cancer survivors and patients – a cluster randomized controlled trial embedded in a mixed methods design. BMC Cancer. 2018;18(1):927. https://doi.org/10.1186/s12885-018-4839-y .
Paszat L, Sutradhar R, O’Brien MA, et al. BETTER HEALTH: Durham – protocol for a cluster randomized trial of BETTER in community and public health settings. BMC Public Health. 2017;17(1):754. https://doi.org/10.1186/s12889-017-4797-3 .
Lofters AK, O’Brien MA, Sutradhar R, et al. Building on existing tools to improve chronic disease prevention and screening in public health: a cluster randomized trial. BMC Public Health. 2021;21(1):1496. https://doi.org/10.1186/s12889-021-11452-x .
Article CAS PubMed PubMed Central Google Scholar
Roberts KC, Rao DP, Bennett TL, Loukine L, Jayaraman GC. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can. 2015;35(6):87–94. https://doi.org/10.24095/hpcdp.35.6.01 .
Campbell-Scherer D, Rogers J, Manca D, et al. Guideline harmonization and implementation plan for the BETTER trial: Building on Existing Tools to Improve Chronic Disease Prevention and Screening in Family Practice. CMAJ Open. 2014;2(1):E1–10. https://doi.org/10.9778/cmajo.20130040 .
Covidence systematic review software. https://www.covidence.org . Melbourne, Australia. Computer program
Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J. 2010;182(18):E839–42. https://doi.org/10.1503/cmaj.090449 .
Article Google Scholar
BC Guidelines. Guidelines and Protocols Advisory Committee Handbook: How our “Made in BC” Clinical Practice Guidelines and Protocols are Developed . Vancouver: British Columbia Ministry of Health;2017. gpachandbook2017.pdf (gov.bc.ca); Accessed Mar 20, 2024.
Alberta Health Services CancerControl Alberta. Guideline Methodology Handbook – Version 5 . 2020. GURU Handbook (albertahealthservices.ca) Accessed Mar 20, 2024.
Canadian Task Force on Preventive Health Care (CTFPHC). Canadian Task Force on Preventive Health Care Procedure Manual . Public Health Agency of Canada (PHAC);2014. https://canadiantaskforce.ca/methods/ Accessed Jan 23, 2022.
Davino-Ramaya C, Krause LK, Robbins CW, et al. Transparency Matters: Kaiser Permanente’s National Guideline Program Methodological Processes. Perm J. 2012;16(1):55–62. https://doi.org/10.7812/TPP/11-134 .
Michigan Quality Improvement Consortium (MQIC). MQIC Guideline Development Criteria . 2013. Clinical Care Guidelines | University of Michigan Health (uofmhealth.org) Accessed Jan 23, 2022.
National Institute for Health and Care Excellence (NICE). Developing NICE guidelines: the manual. Manchester: National Institute for Health and Care Excellence; 2014.
Toward Optimized Practice (TOP). Toward Optimized Practice Clinical Practice Guideline Development Methodology. 2016. https://actt.albertadoctors.org/cpgs Accessed May 25, 2020.
University of Michigan. Michigan Medicine Quality Department Clinical Care Guidelines: Purpose and Methods. 2019. https://www.uofmhealth.org/provider/clinical-care-guidelines . Accessed May 25 2020. Accessed Mar 20, 2024.
World Health Organization (WHO). WHO handbook for guideline development, 2nd Edition. Geneva: World Health Organization, WHO Library Cataloguing-in-Publication Data; 2014.
Wingood GM, DiClemente RJ. The ADAPT-ITT Model. JAIDS J Acqu Immune Deficiency Syndromes. 2008;47(Supplement 1):S40–6. https://doi.org/10.1097/QAI.0b013e3181605df1 .
Article PubMed Google Scholar
Watson P. How to screen for ACEs in an efficient, sensitive, and effective manner. Paediatr Child Health. 2019;24(1):37–8. https://doi.org/10.1093/pch/pxy146 .
California Surgeon General’s Clinical Advisory Committee. Adverse Childhood Experience Questionnaire for Adults. California: Office of the Surgeon General/California Department of Health Care Services; 2020.
BC Guidelines. Problem Drinking. 2013. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/problem-drinking . Accessed May 29, 2020.
Curry SJ, Krist AH, Owens DK, et al. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults. JAMA. 2018;320(18):1899. https://doi.org/10.1001/jama.2018.16789 .
National Institute for Health and Care Excellence (NICE). [CG115] Alcohol-use disorders: diagnosis, assessment and management of harmful drinking (high-risk drinking) and alcohol dependence 2011. Overview | Alcohol-use disorders: diagnosis, assessment and management of harmful drinking (high-risk drinking) and alcohol dependence | Guidance | NICE, Accessed Mar 20, 2024.
Canadian Centre on Substance Abuse and Addiction (CCSA). Guidelines for Healthcare Providers to Promote Low-Risk Drinking Among Patients . 2013. www.ccsa.ca/Resource Library/2012-Canada-Low-Risk-AlcoholDrinking-Guidelines-Poster-en.pdf . Accessed May 25, 2020.
UK Chief Medical Officers (CMO). UK Chief Medical Officers’ Low Risk Drinking Guidelines 2016. https://www.gov.uk/government/consultations/health-risks-from-alcohol-new-guidelines . Accessed May 28, 2020
Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1. https://doi.org/10.1186/1471-244X-14-S1-S1 .
Gregory KD, Chelmow D, Nelson HD, et al. Screening for Anxiety in Adolescent and Adult Women: A Recommendation From the Women’s Preventive Services Initiative. Ann Intern Med. 2020;173(1):48–56. https://doi.org/10.7326/M20-0580 .
Cancer Care Ontario. Magnetic Resonance Imaging Screening of Women at High Risk for Breast Cancer. 2018. https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/screening/breast-cancer-high-risk-women/faqs-for-healthcare-providers . 2018. Accessed May 25, 2020.
Towards Optimized Practice. Breast Cancer Screening Clinical Practice Guideline. 2013. https://actt.albertadoctors.org/CPGs/Lists/CPGDocumentList/Breast-Cancer-Screening-CPG.pdf . Accesses May 29, 2020
Eastern Health. Breast Magnetic Resonance Imaging (MRI) and High Risk Hereditary Breast Cancer. 2017 https://www.easternhealth.ca/wp-content/uploads/sites/2/2018/06/Breast-Magnetic-Resonance-MRI-and-High-Risk-Guideline_2017.pdf Accessed May 25, 2020.
Eastern Health. Indications for Use of Breast Magnetic Resonance Imaging (MRI). 2018. https://www.easternhealth.ca/wp-content/uploads/sites/2/2018/06/Indications_for_Use_of_Breast_Magnetic_Imaging_MRI_Jan_2018.pdf Accessed May 25, 2020.
Fischer B, Russell C, Sabioni P, et al. Lower-Risk Cannabis Use Guidelines: A Comprehensive Update of Evidence and Recommendations. Am J Public Health. 2017;107(8):e1–12. https://doi.org/10.2105/AJPH.2017.303818 .
Toward Optimized P. Cervical Cancer Screening Clinical Practice Guideline. 2016. cervical-cancer-screening-cpg.pdf (albertadoctors.org) Accessed May 25, 2020.
Dickinson J, Tsakonas E, Conner Gorber S, et al. Recommendations on screening for cervical cancer. CMAJ. 2013;185(1):35–45. https://doi.org/10.1503/cmaj.121505 .
Cancer Care Ontario. Ontario Cervical Screening Guidelines Summary. Toronto: Cancer Care Ontario; 2016.
Toward Optimized Practice (TOP) Working Group for Colorectal Cancer Screening. Colorectal Cancer Screening Clinical Practice Practice Guideline. Edmonton: Accelerating Change Transformation Team (ACCT) Alberta Medical Association; 2013.
Provenzale D, Ness RM, Llor X, et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 2.2020. J Nat Comprehen Cancer Net. 2020;18(10):1312–1320. https://doi.org/10.6004/jnccn.2020.0048 .
Scottish Intercollegiate Guidelines Network (SIGN). SIGN 126 Diagnosis and management of colorectal cancer. 2016. https://www.sign.ac.uk/media/1064/sign126.pdf Accessed Mar 20, 2024.
Leddin D, Lieberman DA, Tse F, et al. Clinical Practice Guideline on Screening for Colorectal Cancer in Individuals With a Family History of Nonhereditary Colorectal Cancer or Adenoma: The Canadian Association of Gastroenterology Banff Consensus. Gastroenterology. 2018;155(5):1325-1347.e3. https://doi.org/10.1053/j.gastro.2018.08.017 .
Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188(5):340–8. https://doi.org/10.1503/cmaj.151125 .
Article PubMed Central Google Scholar
Cancer Care Ontario. Colorectal Cancer. 2019 https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/screening/resources-healthcare-providers Accessed Mar 20, 2024
U. S. Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. 2016;315(23):2564–2575. Recommendation: Colorectal Cancer: Screening | United States Preventive Services Taskforce ( uspreventiveservicestaskforce.org ). Accessed 20 Mar 2024.
Black A, Guilbert E, Costescu D, et al. Canadian Contraception Consensus. J Obstet Gynaecol Can. 2015;37(11):1033–5. https://doi.org/10.1016/s1701-2163(16)30054-8 .
BC Guidelines. Hypertension – Diagnosis and Management. 2020. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/hypertension . Accessed Mar 20, 2024.
Lindsay P, Connor Gorber S, Joffres M, et al. Recommendations on screening for high blood pressure in Canadian adults. Can Fam Physician . 2013;59(9):927–933, e393–400.
Siu AL, U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–86 https://doi.org/10.7326/M15-2223 .
Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada’s 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2020;36(5):596–624. https://doi.org/10.1016/j.cjca.2020.02.086 .
National Institute for Health and Care Excellence (NICE). Depression in adults: recognition and management : guidance (CG90). National Institute for Health and Care Excellence - NICE; 2009/10/28 2009. https://www.nice.org.uk/guidance/ng222 . Accessed May 25, 2020.
Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(4):380–7. https://doi.org/10.1001/jama.2015.18392 .
Article CAS PubMed Google Scholar
Joffres M, Jaramillo A, Dickinson J et al. Canadian Task Force for Preventive Health Care (CTFPHC). Recommendations on screeming for depression in adults . CMAJ 2013; 185(9):775–782. https://doi.oorg/ https://doi.org/10.1503/cmaj . 130403
Institute for Clinical Systems Improvement (ICSI). A dult Depression in Primary Care. 2016. https://www.icsi.org/wp-content/uploads/2021/11/Depr.pdf Accessed Mar 20, 2024.
Diabetes Canada Clinical Prractice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Prractice Guidelines for the Prevention and Management of Diabates in Canada. Can J Diabetes. 2018;42(Suppl1):S1–325.
Google Scholar
Pottie K, Jaramillo A, Lewin G, et al. Recommendations on screening for type 2 diabetes in adults. CMAJ. 2012;184(15):1687–96. https://doi.org/10.1503/cmaj.120732 .
Siu AL, U S Preventive Services Task Force. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163(11):861–8 https://doi.org/10.7326/M15-2345 .
ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131(2):e49–64. https://doi.org/10.1097/AOG.0000000000002501 .
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–31 https://doi.org/10.2337/dc20-S002 .
Wilson RD, Genetics Committee, Wilson RD, et al. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. J Obstet Gynaecol Can. 2015;37(6):534–52 https://doi.org/10.1016/s1701-2163(15)30230-9 .
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(2):183–9 https://doi.org/10.1001/jama.2016.19438 .
Health Canada. Canada’s Dietary Guidelines. 2018. CDG-EN-2018.pdf (canada.ca) Accessed Mar 20, 2024.
Nutrition Working Group, O’Connor DL, Blake J, et al. Canadian Consensus on Female Nutrition: Adolescence, Reproduction, Menopause, and Beyond. J Obstet Gynaecol Can. 2016;38(6):508-554.e18 https://doi.org/10.1016/j.jogc.2016.01.001 .
Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2016;32(11):1263–82. https://doi.org/10.1016/j.cjca.2016.07.510 .
Toward Optimized Practice. Prevention and Risk Management of Cardiovascular Disease Risk in Primary Care Clinical Practice Guideline . 2015. https://actt.albertadoctors.org/media/b21chzfk/cvd-risk-cpg.pdf . Accessed Man 20, 2024.
U.S. Preventive Services Task Force (USPSTF). Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA :J Am Med Assoc. 2017;318(2):167–74 https://doi.org/10.1001/jama.2017.7171 .
Ross R, Chaput JP, Giangregorio LM, et al. Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45(10 (Suppl. 2)):S57-S102. https://doi.org/10.1139/apnm-2020-0467
Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81. https://doi.org/10.1093/eurheartj/ehw106 .
National Institute for Health and Care Excellence (NICE). Cardiovascular disease: risk assessment and reduction, including lipid modification – NICE guideline . Royal College of Physicians of London - RCP; 2014/07/01 2016. https://www.nice.org.uk/guidance/ng238 . Accessed May 25, 2020.
Allan GM, Lindblad AJ, Comeau A, et al. Simplified lipid guidelines: Prevention and management of cardiovascular disease in primary care. Can Fam Phys. 2015;61(10):857–67 e439–50.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Risk Reduction version 1.2020. https://www.nccn.org/guidelines/category 1. Accessed May 25, 2020.
Public Health Agency of Canada. Rubella vaccine: Canadian Immunization Guide. 2016; https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-20-rubella-vaccine.html . Accessed June 4, 2020.
Public Health Agency of Canada. Amendment to the 2015 “Update on the recommended Human Papillomavirus (HPV) vaccine immunization schedule.” Ottawa, Ontario: Public Health Agency of Canada; 2015.
Public Health Agency of Canada. Canadian immunization guide. Part 3. Vaccination of specific populations. 2016. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations.html . Accessed 4 June 2020.
Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. Can Med Assoc J. 2020;192(31):E875–91. https://doi.org/10.1503/cmaj.191707 .
Brauer P, Gorber SC, Shaw E, et al. Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. CMAJ. 2015;187(3):184–95. https://doi.org/10.1503/cmaj.140887 .
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Behavioral Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(11):1163–71 https://doi.org/10.1001/jama.2018.13022 .
Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. https://doi.org/10.1161/01.cir.0000437739.71477.ee .
Fitch A, Everling L, Fox C, et al. Institute for Clinical Systems Improvement. Prevention and Management of Obesity for Adults . . Updated May 2013. https://1library.net/document/y69pmp7y-prevention-and-management-of-obesity-for-adults.html . Accessed 20 Mar 2024.
LeFevre ML. U.S. Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;161(8):587–93 https://doi.org/10.7326/M14-1796 .
Public Health England (PHE). Delivering better oral health: an evidence-based toolkit for prevention. 2017. Delivering better oral health: an evidence-based toolkit for prevention - GOV.UK (www.gov.uk ) Accessed Mar 20, 2024.
Kavan MG, Saxena SK, Rafiq N. General Parenting Strategies: Practical Suggestions for Common Child Behavior Issues. Am Fam Physician. 2018;97(10):642–8.
PubMed Google Scholar
Alberta Health Services. Nutrition Guideline Household Food Insecurity . 2013. https://www.albertahealthservices.ca/assets/info/nutrition/if-nfs-ng-household-food-insecurity.pdf Accessed Mar 20, 2024.
Pottie K, Kendall CE, Aubry T, et al. Clinical guideline for homeless and vulnerably housed people, and people with lived homelessness experience. CMAJ. 2020;192(10):E240–54. https://doi.org/10.1503/cmaj.190777 .
Moser A, Stuck AE, Silliman RA, Ganz PA, Clough-Gorr KM. The eight-item modified Medical Outcomes Study Social Support Survey: psychometric evaluation showed excellent performance. J Clin Epidemiol. 2012;65(10):1107–16. https://doi.org/10.1016/j.jclinepi.2012.04.007 .
Public Health Agency of Canada (PHAC). Sexually transmitted and blood-borne infections: guides for health professionals. Canadian Guidelines on Sexually Transmitted Infections. Ottawa: Public Health Agency of Canada; 2020.
LeFevre ML, U.S. Preventive Services Task Force. Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902–910. https://doi.org/10.7326/M14-1981
Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 2015;64(RR-03):1–137.
Kaiser P. Sexually Transmitted Infection: Screening, Testing and Treatment Guideline . 2019. https://wa-provider.kaiserpermanente.org/provider-manual/patient-care/clinical-guidelines Accessed May 25, 2020.
Siu AL. U.S. Preventive Services Task Force. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163(8):622–34 https://doi.org/10.7326/M15-2023 .
Thombs BD, Jaramillo Garcia A, Reid D, et al. Recommendations on behavioural interventions for the prevention and treatment of cigarette smoking among school-aged children and youth. CMAJ. 2017;189(8):E310–6. https://doi.org/10.1503/cmaj.161242 .
CAN-ADAPTT. Canadian Smoking Cessation Clinical Practice Guideline . Toronto, Canada: Canadian Action Network for the Advancement, Dissemination and Adoption of Practice-informed Tabacco Treatment, Centre for Addiction and Mental Health. 2011. The first Canadian Guidelines for Tobacco Control was developed in 2010 (utoronto.ca) Accessed Mar 20, 2024.
Registered Nurses’ Association of Ontario. Engaging Clients Who Use Substances . 2015. https://rnao.ca/sites/rnao-ca/files/Engaging_Clients_Who_Use_Substances_WEB.pdf Accessed Mar 20, 2024.
US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Unhealthy Drug Use: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(22):2301–9 https://doi.org/10.1001/jama.2020.8020 .
Ontario Health Cancer Care Ontario. Vaping products including e-cigarettes: Evidence summary . Ontario, Canada: Ontario Health (Cancer Care Ontario); 2020. https://www.cancercareontario.ca/en/content/vaping-products-including-e-cigarettes . Accessed Mar 20, 2024.
Livingston CJ, Freeman RJ, Costales VC, et al. Electronic Nicotine Delivery Systems or E-cigarettes: American College of Preventive Medicine’s Practice Statement. Am J Prev Med. 2019;56(1):167–78. https://doi.org/10.1016/j.amepre.2018.09.010 .
Shimizu T, Bouchard M, Mavriplis C. Update on age-appropriate preventive measures and screening for Canadian primary care providers. Can Fam Physician. 2016;62(2):131–8.
PubMed PubMed Central Google Scholar
Ridley J, Ischayek A, Dubey V, Iglar K. Adult health checkup: Update on the Preventive Care Checklist Form©. Can Fam Physician. 2016;62(4):307–13.
Persaud N, Sabir A, Woods H, et al. Preventive care recommendations to promote health equity. Can Med Assoc J. 2023;195(37):E1250–73. https://doi.org/10.1503/cmaj.230237 .
Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice (Red Book). Melbourne: Royal Australian College of General Practitioners; 2021. http://Guidelinesfor-preventive-activities-in-general-practice.pdf ( http://racgp.org.au ). Accessed 20 Mar 2024.
Sundaram ME, Calzavara A, Mishra S, et al. Individual and social determinants of SARS-CoV-2 testing and positivity in Ontario, Canada: a population-wide study. Can Med Assoc J. 2021;193(20):E723–34. https://doi.org/10.1503/cmaj.202608 .
Article CAS Google Scholar
Toronto Public Health. COVID-19 and the Social Determinants of Health: What do we know? 2020. 96e0-SDOHandCOVID19_Summary_2020May14.pdf (toronto.ca). Accessed 20 Mar 2024.
Download references
Acknowledgements
Carolyn Ziegler for the systematic search. Jane Ebreo for all administrative support. Kimberly Devotta for project management support. Tutsirai Makuwaza for feedback on the qualitative work on BETTER LIFE. Mary-Anne Pietrusiak for subject matter expertise. Ranya Mistry for Qualtrics survey development support.
This study was funded by a Canadian Institutes of Health Research Catalyst Grant: Disease Prevention and Risk Factor Modification – Non-Communicable Diseases (#428589).
Author information
Mary Ann O’Brien and Aisha Lofters are co-senior authors.
Authors and Affiliations
Public Health Agency of Canada, Ottawa, ON, Canada
Nasheed Moqueet
McMaster University, Hamilton, ON, Canada
Sylvie D. Cornacchi
Women’s College Hospital, 76 Grenville St, Toronto, ON, M5S 1B2, Canada
Jesmin Antony & Aisha Lofters
Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, ON, Canada
Ielaf Khalil
Department of Family Medicine, University of Alberta, Edmonton, AB, Canada
Donna Manca, Carolina Fernandes, Nicolette Sopcak & Jill Konkin
Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Lawrence Paszat
Primary Healthcare Research Unit, Memorial University of Newfoundland, St. John’s, NL, Canada
Kris Aubrey-Bassler
Ontario Institute for Cancer Research, Toronto, ON, Canada
Eva Grunfeld
Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON, Canada
Andrew Pinto
School of Public Health, University of Alberta, Edmonton, AB, Canada
Candace Nykiforuk
Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada
Linda Rabeneck
Centre for Addiction and Mental Health, Toronto, ON, Canada
Peter Selby
Department of Family and Community Medicine, University of Toronto, Toronto, ON, Canada
Eva Grunfeld, Peter Selby, Mary Ann O’Brien & Aisha Lofters
Durham Region Health Department, Whitby, ON, Canada
You can also search for this author in PubMed Google Scholar
Contributions
NM, SDC, JA, IK, MAO, AL provided substantial contributions to the conception and design of the work (review, data synthesis, data extraction, quality assessment, harmonization); NM, SDC, JA, IK, DM, CF, LP, PS, MAO, AL acquired, analyzed, and interpreted data; NM, SDC, IK, MAO, AL wrote the manuscript;NM, SDC, JA, IK, DM, CF, LP, KAB, EG, NS, AP, JK, CN, LR, PS, BW, MAO, AL (i.e. all authors) reviewed the manuscript critically for important intellectual content; NM, SDC, JA, IK, DM, CF, LP, KAB, EG, NS, AP, JK, CN, LR, PS, BW, MAO, AL (i.e. all authors) read and approved the final manuscript.
Corresponding author
Correspondence to Aisha Lofters .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., supplementary material 4., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Moqueet, N., Cornacchi, S.D., Antony, J. et al. BETTER LIFE- guidelines for chronic disease preventive care for people aged 18–39 years: a literature review. BMC Prim. Care 25 , 224 (2024). https://doi.org/10.1186/s12875-024-02471-9
Download citation
Received : 06 September 2023
Accepted : 10 June 2024
Published : 22 June 2024
DOI : https://doi.org/10.1186/s12875-024-02471-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health promotion
BMC Primary Care
ISSN: 2731-4553
- General enquiries: [email protected]
Additional Options
- smartphone Call / Text
- voice_chat Consultation Appointment
- place Visit
- email Email
Chat with a Specific library
- College Library (Undergraduate) College Library Chat is Offline
- Ebling Library (Health Sciences) Ebling Library Chat is Offline
- Law Library (Law) Law Library Chat is Offline
- MERIT Library (Education) MERIT Library Chat is Offline
- Ask a Librarian Hours & Policy
- Library Research Tutorials
Search the for Website expand_more Articles Find articles in journals, magazines, newspapers, and more Catalog Explore books, music, movies, and more Databases Locate databases by title and description Journals Find journal titles UWDC Discover digital collections, images, sound recordings, and more Website Find information on spaces, staff, services, and more
Language website search.
Find information on spaces, staff, and services.
- ASK a Librarian
- Library by Appointment
- Locations & Hours
- Resources by Subject
book Catalog Search
Search the physical and online collections at UW-Madison, UW System libraries, and the Wisconsin Historical Society.
- Available Online
- Print/Physical Items
- Limit to UW-Madison
- Advanced Search
- Browse by...
collections_bookmark Database Search
Find databases subscribed to by UW-Madison Libraries, searchable by title and description.
- Browse by Subject/Type
- Introductory Databases
- Top 10 Databases
article Journal Search
Find journal titles available online and in print.
- Browse by Subject / Title
- Citation Search
description Article Search
Find articles in journals, magazines, newspapers, and more.
- Scholarly (peer-reviewed)
- Open Access
- Library Databases
collections UW Digital Collections Search
Discover digital objects and collections curated by the UW Digital Collections Center .
- Browse Collections
- Browse UWDC Items
- University of Wisconsin–Madison
- Email/Calendar
- Google Apps
- Loans & Requests
- Poster Printing
- Account Details
- Archives and Special Collections Requests
- Library Room Reservations
Search the UW-Madison Libraries
Article search, preventive healthcare behavior: a hybrid systematic literature review (1998–2023).
- Preventive healthcare behavior (PHB) refers to actions taken by consumers to avert possible incidences of lifestyle diseases. Over the years, diverse approaches have been used to comprehend the complex nature of PHB. This paper follows a three‐step process to examine work done in the PHB domain. At the outset, past literature was examined. This review included PHB models and frameworks, followed by a root‐cause analysis to identify factors that impacted PHB adoption. A systematic literature review (SLR) using a domain‐based hybrid review approach was the study's third and most crucial part. The SPAR‐4‐SLR (scientific procedures and rationales for systematic literature reviews) protocol was used to conduct the hybrid review, involving two separate review studies. In the first study, a bibliometric analysis was carried out, wherein a trend analysis was conducted on an initial pool of 1011 primary peer‐reviewed publications (1998–2023). The trend analysis was followed by a co‐citation network analysis of 39,608 secondary articles, which validated the importance of primary articles as indicated by the co‐citations in these secondary articles. Further, a purification process based on reliability, validity, and replicability criteria resulted in a final pool of 190 relevant articles. These articles were subjected to a thematic analysis. Next, a framework‐based review based on the theories, contexts, characteristics, and methods (TCCM) framework was conducted on the 190 articles. This step validated the primary study findings. Additionally, it examined and reported the underlying theories, context (country level), characteristics, and methods adopted by previous PHB studies. This analysis helps in indicating the future research agenda regarding PHB. Furthermore, the inferences drawn from the two studies were used to propose a conceptual framework for understanding consumers' PHB decisions based on the antecedents, decisions, and outcomes (ADO) framework. The framework posits that specific personal, demographic, cultural, social and socioeconomic factors are precursors to PHB adoption. This PHB adoption, in turn, has positive outcomes such as enhanced quality of life, consumer wellbeing, health promotion, health behavior change, and planning. The comprehensive review and proposed framework will significantly help advance knowledge about PHB. Apart from contributing to the academic literature, learnings from this study hold value for practitioners at the global level for designing actionable strategies for preventive healthcare products and services.
- View online access
Having Trouble?
If you are having trouble accessing the article, report a problem.
Physical Copies
Publication details.
- Wiley Online Library
- ADO framework
- Behavior change
- bibliometric analysis
- Bibliometrics
- Frame analysis
- Health behavior
- Health care
- Health promotion
- Literature reviews
- Network analysis
- preventive healthcare behavior
- Preventive medicine
- Purification
- Quality of life
- Reliability
- Socioeconomic factors
- systematic literature review
- Systematic review
- TCCM framework
Additional Information
Library staff details, keyboard shortcuts, available anywhere, available in search results.

IMAGES
COMMENTS
The goal of this systematic literature review was to update the study by Balas et al., 17 which included 16 preventive care measures from the US Preventive Task Force, and to examine whether the amount of computerized reminder systems for preventive care have changed as clinicians increasingly utilize electronic health record systems when ...
Find methods information, sources, references or conduct a literature review on PREVENTIVE MEDICINE. Science topics: Medicine Public Health Preventive Medicine. Science topic.
Sep 27, 2024 · Background Action on smoking, obesity, excess alcohol, and physical inactivity in primary care is effective and cost-effective, but implementation is low. The aim was to examine the effectiveness of strategies to increase the implementation of preventive healthcare in primary care. Methods CINAHL, CENTRAL, The Cochrane Database of Systematic Reviews, Dissertations & Theses – Global, Embase ...
highlighted the importance of preventive medicine in protecting public health and has led to an increased emphasis on vaccination and other preventive measures. This literature review aims to explore how primary and secondary prevention is being done in different countries worldwide. The review will focus on primary health care, vaccination, and
Chou R, Dana T, Blazina I, Daeges M, and Jeanne TL. 2016. “Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force.” Jama 316 (19): 2008–2024. [Google Scholar] Clarke EA 1974. “What is Preventive Medicine?” Can Fam Physician 20 (11): 65–8.
aims to explore the role of preventive medicine in enhancing public health outcomes, with a specific focus on the critical importance of routine health screenings in early disease detection and prevention. Methods: A comprehensive literature review was conducted, analyzing peer-reviewed articles, public health data, and case studies on the
Key words: preventive medicine, clinical practice guidelines, methodology, health equity. INTRODUCTION. The US Preventive Services Task Force (USPSTF) is an independent body formed in 1984 to make evidence-based recommendations regarding preventive services including screening, behavioral counseling, and preventive drugs.
This paper explores the significance of preventive medicine in promoting wellness and improving health outcomes across populations. Through an extensive literature review, key components of preventive medicine, including immunizations, screenings, lifestyle interventions and public health initiatives, are examined.
Jun 22, 2024 · Overview of search strategy. First, we assessed the data sources (clinical practice guidelines) from the most recent BETTER WISE study [], which had entailed a rigorous evidence review process to recommend specific prevention and screening actions, for applicability to adults aged 18–39 years.Then, we used a structured grey literature search of specific repositories and websites to find ...
The comprehensive review and proposed framework will significantly help advance knowledge about PHB. Apart from contributing to the academic literature, learnings from this study hold value for practitioners at the global level for designing actionable strategies for preventive healthcare products and services.